
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Draw the pi system orbitals of the following molecule.
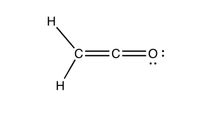
Transcribed Image Text:H
C=C= 0:
H
•.
O:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Nonearrow_forwardChoose the selection which correctly identifies the sets of hybrid orbitals used by central atoms to form bonds in both of the molecules and/or ions whose formulas are given below:ICl2- and BH3 a) The central atom of ICl2- uses sp3d2 hybridization and the central atom of BH3 uses sp2 hybridization.b) The central atom of ICl2- uses sp3d hybridization and the central atom of BH3 uses sp3d hybridization.c) The central atom of ICl2- uses sp3d hybridization and the central atom of BH3 uses sp3 hybridization.d) The central atom of ICl2- uses sp hybridization and the central atom of BH3 uses sp3 hybridization.e) The central atom of ICl2- uses sp3d hybridization and the central atom of BH3 uses sp2 hybridization.arrow_forward1. Determine the highest order proper axis of rotation for each of the following geometric shapes.arrow_forward
- can you please explain the configuration? for the triangle structure i got s but I am lost.arrow_forwardWhat would be an example of a molecule that contains only one sp hybridized atom (it can contain many atoms, but only one of them can be sp hybridized)?arrow_forwardWhen an atomic s orbital hybridizes with three atomic p orbitals, the atom will have: Group of answer choices three hybrid orbitals and one unhybridized p orbital. three sp3 orbitals and no unhybridized p orbitals. one hybridized orbital and two unhybridized p orbitals. four hybridized orbitals.arrow_forward
- Match the given molecular formula with the expected hybridization about the central atom. HCN, SO2, H2O, CIF3, IF5 sp3d, sp3, sp3d2, sp, sp2arrow_forwardUse the References to access important values if needed for this question. Answer the following questions about the hybrid orbitals on the chlorine atom in the ClO3+ and ClO2+ molecular ions. What are the expected geometries of these ions? ClO3+: Central atom = Cl Type of hybrid orbitals on the chlorine atom = Number of hybrid orbitals used in overlap with atomic orbitals = Number of hybrid orbitals accomodating unshared electrons = Hybrid orbital geometry = Molecular geometry = ClO2+: Central atom = Cl Type of hybrid orbitals on the chlorine atom = Number of hybrid orbitals used in overlap with atomic orbitals = Number of hybrid orbitals accomodating unshared electrons = Hybrid orbital geometry = Molecular geometry =arrow_forwardWhich molecule contains sp hybridized orbitals?arrow_forward
- Can you explain how to draw hybridization diagrams? How do I determine the hybrid orbitals? How can I draw the energy diagram? Please explain using an example.arrow_forwardFormula Electron configuration (central atom) and energy level diagram before promotion BC13 Be Br₂ SF6 SiH4 Pls GeF4 Hybridization Worksheet Energy level diagram after electron promotion (include empty orbitals where appropriate) Energy level diagram after hybridization (include empty orbitals where appropriate)arrow_forward4.) Fill in the following table with the number of carbon atoms of the specific hybridization state belonging to the following molecule. CH3 CN Hybridization states of carbon # of carbon atoms sp sp? sparrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY