
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Plz do Asap.....!
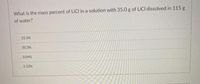
Transcribed Image Text:What is the mass percent of LICI in a solution with 35.0 g of LICI dissolved in 115 g
of water?
C 23.3%
30.3%
3.04%
2.33%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Campuswire Bb Chapter 3 – 2150 Fall 2021 08/ X D (268) COVIDeos lecture series X (268) Deep House Mix 202 1 X Get Homework Help With Chec X C It Is Pertolu: - Blackboard Lex 101 Chem101 + app.101edu.co M Apps G M Gmail YouTube Maps а АМAZON Translate Gflights Case Status Onlin... Reading List Question 15.c of 30 Submit How does the quantization of energy explain the observed photoelectric effect and emission of UV energy? When light shines on a metal, electrons can be ejected from the surface of the metal in “the photoelectric effect". Experiments showed that the wavelength of light is inversely proportional to kinetic energy of the electrons ejected. Identify the best explanation of the photoelectric effect. A) Increasing the brightness of incoming light increases the kinetic energy of the ejected electrons. B) Increasing the wavelength of incoming light increases the kinetic energy of the ejected electrons. C) Increasing the frequency of incoming light increases the kinetic energy of…arrow_forwardMALEKS T LI ALEKS - Rafia Riaz - Learn Type here to search www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lvdw7xgLCkqMfg8yaFKbD9GafJstkYLIJnusptTd4BMWR5tqw8EEwiGTTdyzfqHFomOky9feFge9QQitSAMUOKacSVpwDeo?1... Q = O ELECTROCHEMISTRY Using the Nernst equation to calculate nonstandard cell voltage X + A galvanic cell at a temperature of 25.0 °C is powered by the following redox reaction: 2+ Sn²+ (aq) + Ba (s) → Sn (s) +Ba²+ (aq) 2+ 2+ Suppose the cell is prepared with 3.24 M Sn in one half-cell and 2.23 M Ba in the other. Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. 0 Explanation 발 Check x10 ロ・ロ Olo 0/3 Rafia V ? 圖 □ □ 图 olo Ar © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility (?) 46°F Mostly clear * 9:09 AM 5/5/2023 x : Marrow_forwardon Commun x Bb Courses- Blackboard Learn > General Psychology-Fall 2021 - x A ALEKS - Griffin Barden - Learn ww-awa.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IJcZZdcvSCzsqTCIDqNGV3bKqhMfPmUcQ4ENkmiXn9QCwgeDPDkQ06yszYWEsPcekwL0-Qg619rekU7404HgFAGbEZaDr080?1oBw7QYjlbavbSPXtx-YCjsh_7mMm O THERMOCHEMISTRY Griffin Calculating specific heat capacity A chemist carefully measures the amount of heat needed to raise the temperature of a 860.0 g sample of a pure substance from 14.8 °C to 22.4 °C. The experiment shows that 4.6 kJ of heat are needed. What can the chemist report for the specific heat capacity of the substance? Round your answer to 2 significant digits. -1 - 1 x10 J'g K X.arrow_forward
- I just need a little help on how to do this the explanations aren't helping me and I'm lost.arrow_forwardAsk Laftan Anlamaz - Episode St. John's University - My App X A ALEKS - Iffat Khan - Learn G In valence electrc -> A www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lijkPWvZoZLgkt1FLIq7wcPWKzBYGfE9IMFj8s1-UE-m2k5S O MATTER Finding the side length of a cube from its volume in liters A technical machinist is asked to build a cubical steel tank that will hold 390 L of water. Calculate in meters the smallest possible inside length of the tank. Round your answer to the nearest 0.01 m. Omarrow_forward2. Please deduce the following fundamental equations. TdS -PdV www du www =arrow_forward
- n Course: CHEM262-SEC01 Org x V OWL Exam O Noor Abid - 32843659 S x b Success Confirmation of Quest x G how to take a screenshot on m + A owl.umass.edu/owlj/servlet/Student Update : M Gmail O YouTube O Google Keep n UMass Amherst M.. c Write The Comple... O SPIRE Logon A Swank Movies O Discovering Nutrit. 26 UMass Amherst -. t. Minitab >> Select the major product of the following reactions. 1. NaOEt 2. CH3I EtO OEt 3. NaOEt 4. CH3I 5. H,0*/ heat Is 6. CH,ОН/ Н+ HO, OH IV I II III O None of the choices O IV O IIarrow_forwardChapter 12arrow_forwardto Johnston Commun X Rh Blackboard Collaborate Ultra - x > General Psychology-Fall 2021 A ALEKS - Griffin Barden - Learn + A www-awa.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IJczzdcvSCzsqTCIDqNGV3bKqhMfPmUcQ4ENkmiXn9QCwgeDPDkQ06yszYWESPcekwLO-Qg619rekU7404HgFAGbEZa Dr080?1 oBw7QYjlbavbSPXtx-YCjsh_7mMmrq#item O THERMOCHEMISTRY Griffin Calculating a molar heat of reaction from formation enthalpies Using the table of standard formation enthalpies that you'll find under the ALEKS Data tab, calculate the reaction enthalpy of this reaction under standard conditions: 2C,H;(g)+70,(9)→4 CO,(g)+6H,O() Round your answer to the nearest kJ.arrow_forward
- Can you please answer it , has been a hard subject for me . Thank youarrow_forward) 46% Sun 11:04 AM Safari File Edit View History Bookmarks Window Help A session.masteringchemistry.com Ellucian Degree Works... Pearson's MyLab & Mas... ALEKS - Sofia Simmons... MasteringChemistry: H... Consider summer class... Inbox (13) - thesym1@g.. Class Schedule Listing Schedule 111.009 & 111.... View Available Hint(s) Reset Help F2 H2 Mg O2 Oxidizing agent Reducing agent 31410 FEB tv 23 MacBook Pro esc 23 2$ & ....arrow_forward- (17 · (17 Fra Fra ge edu/courses/159861/files?preview=69941104 الولايات المتحدة القن... 0 Ns G Gmail als Science Problem Set 2.pdf W F2 # 2 ۲ 3 r b Ac E 80 F3 D 16 $ Why You Don't Ne... 4 E (PI ASU Fil W Juj (PL R Page of 2 | 0 | 9 al K Û F9 ) O PSU X 0 [ O A Alternative خ F10 . La P * Varrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY