
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:gs- UNF
x ALEKS Chemistry
McGraw-Hill Education Campus
A ALEKS - Lindsay Brooks - Learn
www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-lgNslkr7j8P3jH-IBcWcplLoFLoU71DOb3zrKPTUHJHevE88rwcvGV34AJO-k5zrX83aDiBgWlpQ4wr9EXOY65AO49daviN50MUe5NRg-sH_9k
1 Gmail
YouTube
Maps
O ELECTRONIC STRUCTURE
三I
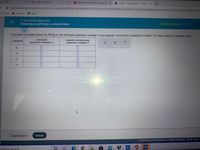
Deducing n and I from a subshell label
Complete the table below by filling in the principal quantum number n and angular momentum quantum number / for each electron subshell listed.
subshell
principal
angular momentum
quantum number n
quantum number /
6p
6f
2s
5f
Explanation
Check
© 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use
%23
APR
o here to search
II
1II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Part A Which of the following combinations of quantum numbers can refer to an electron in a ground-state tellurium atom (2=52)? Check all that apply. O n= 3,1 = 3, m = 0 On=4,1=3, m = 3 On= 3.1= 3. m = -3 Submit Request Answerarrow_forwardidentify the set of quantum numbers that corresponds to an electron in a 3p orbital. Select an answer and submit. For keyboard navigation,, use the up/down arrow keys to select an answer n-1(-3 m, =0 b. n-2/%33 m,-1 n-3(=1m,-0 n-3(-2 m,-1 n=3(-3m,%3D0arrow_forwardPart A What wavelength A should the astrophysicist look for to detect a transition of an electron from the n = 6 to the n = 3 level? Express your answer with the appropriate units. ►View Available Hint(s) A = μA xa Xb Value Submit 06 Previous Answers X.10n X nm X Incorrect; Try Again; 3 attempts remaining You can use either the Rydberg equation ? W = R(-/-)arrow_forward
- Be sure to answer all parts. How X C Choose All Possible Values Of Ea x + nect.mheducation.com/flow/connect.html ework i Saved 2 attempts left Check my work Be sure to answer all parts. Choose all possible values of each quantum number for the outermost electron in an Rb atom. 2 3. 6. 8. 2 2 -4 -3 -2 -1 0. -1 -1/2 0. -1/2 +1 aer 32 Next to searcharrow_forwardPart A Which of the following combinations of n and I represent real orbitals and which are impossible? Drag the appropriate items to their respective bins. Reset Help Real Impossible 3d 1s 4s 3farrow_forwarddo both part , if u plan to do only one part then skip some other will do all,,,, plz give complete solutionarrow_forward
- A.S., Nu. KAssignment: Early Experiments in Quantum Theory • Due in an hour 4/9 answered HQ6.10 Homework • Unanswered O Important Calculate the wavelength of light emitted in the Balmer region, where n = 5. Report your numerical answer in units of nm. R = 1.097 x 107 m-1. Type your numeric answer and submit Unanswered • 2 attempts left A Submit HQ6.11 O Important Homework • Unanswered An orange emission line is observed at 656.3 nm in the hydrogen emission spectrum. Determine the initial energy level of the electron. R= 1.097 x 107 m-1. Type your numeric answer and submit 43°F ^ i Carrow_forwardBro and pro expert Hand written solution is not allowed. Give proper explanation of the correct option and proper explanation of the incorrect options will upvote.arrow_forwardThe energy level diagram below shows the allowed energy levels of an electron in an atom of Element X. Energy (J) | |- C B A -1.1x10-20 -2.3x10-20 -4.9x10-20 Calculate the wavelength of a photon emitted by the transition from C to A. marrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY