
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:IF
ALEKS Chemistry
Ma
Graw
McGraw-Hill Education Campus X A ALEKS - Lindsay Brooks Learn
Chapter 10 Reading Check. Wate X
www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-lgNslkr7j8P3jH-IBcWcplLoFLoU71DOb3zrKPTUHJHevE88rwc--VEA_15anL8CsTNVBMkfKyxkTWNjPG_JZ3ogOpfBu_LMSqSBtHtniGOJ3Eh?
nail
YouTube
Maps
O ELECTRONIC STRUCTURE
Deducing n and I from a subshell label
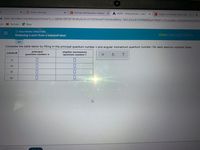
Complete the table below by filling in the principal quantum number n and angular momentum quantum number / for each electron subshell listed.
principal
quantum number n
angular momentum
quantum number /
subshell
3s
3d
4f
4p
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What orbital is described by the following quantum numbers? n = 4 1=3 mi -4 = a 4d orbital these quantum numbers cannot exist a 4s orbital a 4f orbital a 4p orbitalarrow_forwardHere is a sketch of the radial probability distribution of three orbitals: probability 0.0020- 0.0015+ Doc 0.0010- 0.0005- M 500 1000 1500 2000 distance from nucleus (pm) Use this sketch to answer the following questions. What is the most likely distance from the nucleus for an electron in orbital A? Note: your answer must be within 50 pm the exact answer to be graded correct. List the orbitals in order of increasing energy: 2500 Suppose all three orbitals were inner orbitals of an atom. In which orbital would an electron be most effective at screening the charge of the nucleus from the outer electrons? 1000 0 3000 pm (choose one) 0,0,... Śarrow_forwardWhat is the maximum number of electrons that all the orbitals with a principle quantum number of 4 could hold? What about a principle quantum number of 5?arrow_forward
- Use the graphs of the hydrogen 2s and 2p orbitals (probability density) to explain why the energy of the 2s orbital is lower even though it has a higher expectation value for the radiusarrow_forwardIf the principal quantum number is n = 4, what are the name(s) of the subshells? s s, p s, p, d or s, p, d, farrow_forwardWhat quantum numbers specify these subshells? 1s n = I 5p %3D n = 3d Show All 25 SA stv MacBook Airarrow_forward
- What are the values of the principal quantum number and the angular momentum quantum number of the electrons in a 4d subshellarrow_forwardAn electron has the following set of quantum numbers: 3, 2, -1, -1/2 a) What is the energy level for this electron? b) What type of orbital is represented by these quantum numbers? s-orbital p-orbital d-orbital f-orbital c) Which of the following atoms could have an electron in the ground state with these quantum numbers? Ne Ar Ca Krarrow_forwardA subshell with the quantum numbers n = 3, 1 =3 is: O There is no subshell fitting this description O3p O3s O 3darrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY