
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN: 9780134580999
Author: Elaine N. Marieb, Katja N. Hoehn
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
1 . What is the absorption peak(s) of bromocresol green? Make sure to write down the wavelength at which the absorption peak(s) occur. Include units, please
2.Are the absorption spectrums and peak(s) for methyl orange and bromocresol green the same? Why or why not?

Transcribed Image Text:4
3.5
2.5
1.5
0.5
Figure 2 ABSORPTION SPECTRUM OF BROMOCRESOL GREEN
402.5
442.5
462.5
482.5
522.5
542.5
Wavelence (nm)
562.5
582.5
622.5
642.5
662.5
682.5
702.5
722.5
2.
Absorbance
342.5
362.5
382.5
422.5
502.5
602.5
Transcribed Image Text:seño
Disposición
Referencias
Correspondencia
Revisar
Vista
Ayuda
Diseño de
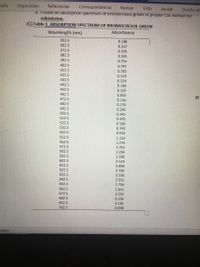
a. Create an absorption spectrum of bromocresol green in proper CSE format for
submission.
2+ Table 1 ABSORPTION SPECTRUM OF BROMOCRESOL GREEN
Wavelength (nm)
Absorbance
352.5
0.186
362.5
0.237
372.5
0.395
382.5
0.595
392.5
0.754
402.5
0.797
412.5
0.703
422.5
0.519
432.5
0.324
442.5
0.180
452.5
0.105
462.5
0.090
472.5
0.116
482.5
0.170
492.5
0.246
502.5
0.341
512.5
0.455
522.5
0.589
532.5
0.740
542.5
0.910
552.5
1.110
562.5
1.376
572.5
1.761
582.5
2.294
592.5
2.930
602.5
3.519
612.5
3.856
622.5
3.789
632.5
3.306
642.5
2.551
652.5
1.736
662.5
1.041
672.5
0.550
682.5
0.256
692.5
0.105
702.5
0.038
hidos)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- You resuspend 1 ml of of a purified chlorophyll sample in 10 ml of 80% acetone, and determine that the optical density of the solution (at 652 nm) is 0.88. What is the concentration of your original preparation?arrow_forwardWhat chemical reaction causes the color change of diphenylamine sulfonate indicator?arrow_forward(please type answer not write by hend)arrow_forward
- 3. Show the calculation of (1) the expected grams of alum (KAI(SO4)2 12 H2O) formed from the reaction of 1.4 grams of aluminum metal and then show the calculation of (2) the % yield if the actual yield of alum is 21.31 grams. Overall reaction is shown below: 2 Al + 2 KOH + 10 H2O + 4 H2SO4 →2 KAI(SO4)2 12 H2O + 3 H2(g)Tarrow_forwardMost of the following conclusions regarding the photo shown above are correct Except: All of the following are correct regarding the disk diffusion test photos EXCEPT : Zone of inhibition Chlorine Chlorine Chlorine O-phenylphenol Hexachlorophene O-phenylphenol Hexachlorophene Hexachlorophene O-phenylpheno Quat Quat Quat Staphylococcus aureus (gram-positive) Escherichia coli Pseudomonas aeruginosa (gram-negative) (gram-negative) Select one: O to. Gram negative bacteria are the most sensitive to chemical agents O b. Only one of the four chemicals affected Pseudomonas O c. Gram positive bacteria are the most sensitive to disinfectants O d. Hexachlorophene was effective only against Gram positive bacteria. O and. Chlorine was effective against all bacteria bacteria testedarrow_forwardBased on the color of the pigments on the chromatography paper, explain which wavelengths of lights are absorbed and reflected by chlorophyll a, chlorophyll b, xanthophyll, and beta carotenearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education