Related questions
Question
11) Complete the structure of thalidomide by drawing multiple bonds
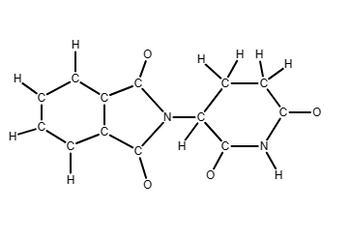
Transcribed Image Text:I
I
H
HI?
H
H
HH
C
H
H
O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 5 images
