
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
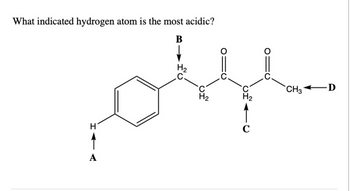
Transcribed Image Text:What indicated hydrogen atom is the most acidic?
B
HAA
fo
OF
VI
C
CH3
·D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify the most acidic compound between the following moleculesDraw the resonance structures of the conjugate base of the acid chosen in (a). Use the curved arrows to show the movement of electrons and the resonance arrows to show the relationship between these structures. All lone electron pairs and charges must be clearly indicatedarrow_forwardWhat indicated hydrogen atom is the most acidic? B O A B D C Cannot tell A H₂ C. C H₂ C CH3 -Darrow_forwardConsider the compound shown below. In consideration of the hydrogen atoms labelled (A, B, & C), the correct ordering for their relative acidities (from most acidic to least acidic is... COH C А НО о с, В, А O A, C, B O C, A, B O None of these are correct. оВ А, С 8arrow_forward
- Which of the following molecules is the most acidic? HỌ CH2 1 4 2 3arrow_forwardactice ! 1 201 FI Q A N 3.4 Brønsted-Lowry Acidity: Factors Affecting the Stability of Anions Considering the hydrogen atoms highlighted in red in the two compounds shown below, which is more acidic and why? 2 A Bos Save for Later W -H OA is the stronger acid because its conjugate base is stabilized by resonance. OB is the stronger acid because its conjugate base is stabilized by resonance. S OA is the stronger acid because the carbon atom bearing the highlighted proton is sp² hybridized. OB is the stronger, acid because the carbon atom bearing the highlighted proton is sp³ hybridized. X # VS. 3 80 F3 E D B $ 4 C 888 F4 R H F % 5 V T G ^ 6 MacBook Air B F6 Y & 7 H F7 U N * 8 J DII FB 1 ( - o M 9 K DD FO O ) Submit Answer O P E FI { [ Aarrow_forwardWhich compound is more acidic?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY