
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
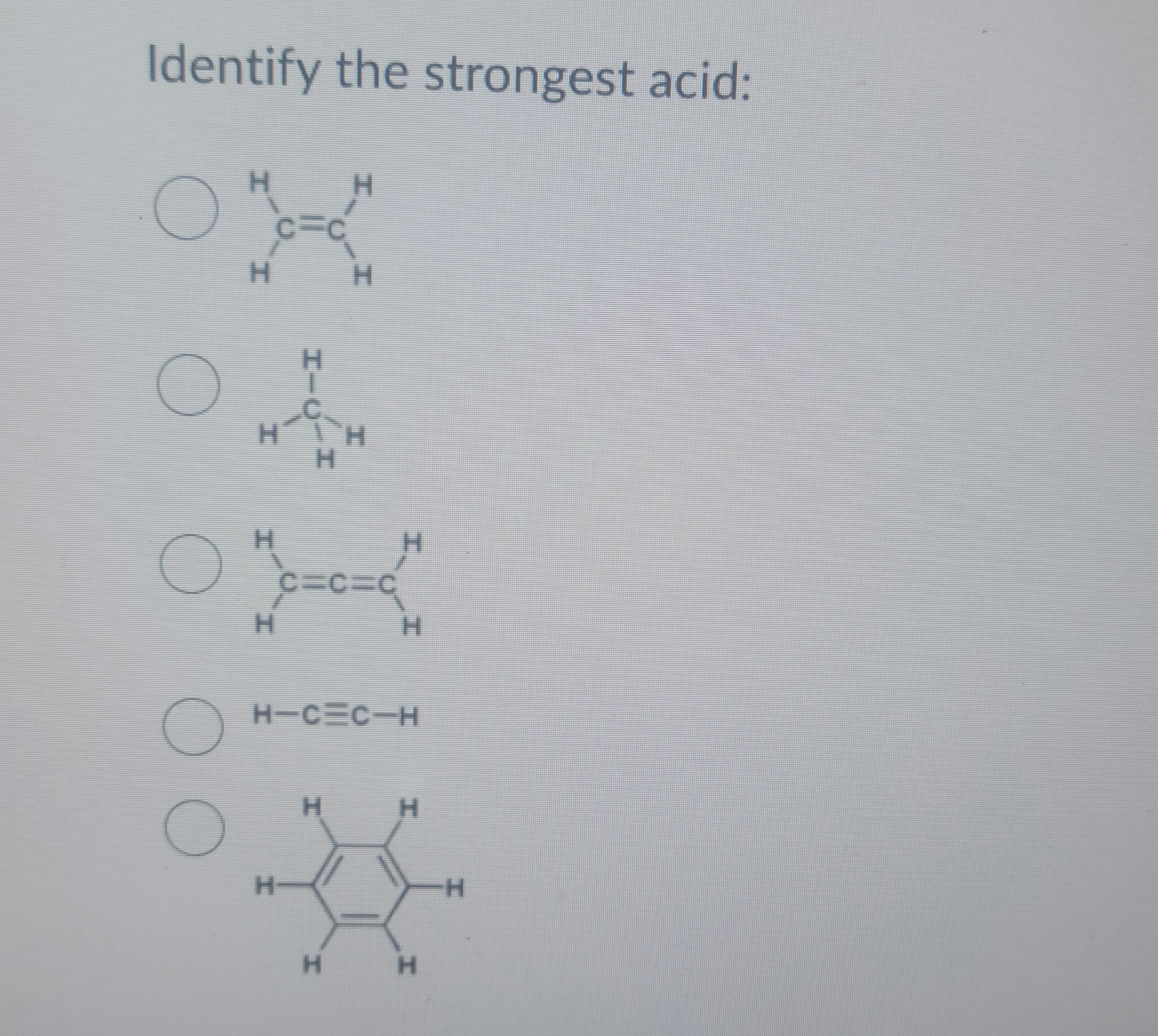
Transcribed Image Text:Identify the strongest acid:
O
O
O
H
C
HÃ
H
H
C-H
H
H-
H
CECEC
H
H-C=C-H
H
H
I
H
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In each row of the table, select the stronger acid or stronger base, as instructed. H H equally acidic Br Select the stronger acid: F Ci equally basic Select the stronger base: F .F -F equally acidic он он Select the stronger acid: Submitarrow_forwardIn the reaction: H₂O + H₂O a strong acid a Brønsted base only O a Brønsted acid only H3O+ + OH, water is acting as neither a Brønsted acid and a Brønsted base both a Brønsted acid and a Brønsted basearrow_forwardFill in the missing chemical formulae in the tables below. acid HNO3 + H₂O* H₂O conjugate base 0 0 0 base 2- CO I HSO4 conjugate acid П 0arrow_forward
- Arrange the following from strongest base to weakest base. F CI Br Select from the possible options A I Br CIF B Cl Br I F Br CI FI FCI Br C D D C B ΘΑarrow_forwardPLEASE ANSWER ASAParrow_forwarddentify the conjugate acid for each base. Conjugate acid of H₂PO4: conjugate acid of SO²: conjugate acid of NH3: APR 9 $ 7 do NH % mtv G Search or type URL MacBook Proarrow_forward
- actice ! 1 201 FI Q A N 3.4 Brønsted-Lowry Acidity: Factors Affecting the Stability of Anions Considering the hydrogen atoms highlighted in red in the two compounds shown below, which is more acidic and why? 2 A Bos Save for Later W -H OA is the stronger acid because its conjugate base is stabilized by resonance. OB is the stronger acid because its conjugate base is stabilized by resonance. S OA is the stronger acid because the carbon atom bearing the highlighted proton is sp² hybridized. OB is the stronger, acid because the carbon atom bearing the highlighted proton is sp³ hybridized. X # VS. 3 80 F3 E D B $ 4 C 888 F4 R H F % 5 V T G ^ 6 MacBook Air B F6 Y & 7 H F7 U N * 8 J DII FB 1 ( - o M 9 K DD FO O ) Submit Answer O P E FI { [ Aarrow_forwardIf lung problems like emphysema occur, the blood pH of the patient is expected to decrease increase stay the same saturate concentratearrow_forwardWhich of the following is the stronger acid? HCOOH (Ka = 1.8 x 104) H2CO3 (Ka = 4.5 x 10-7) HSO4" (Ka = 1x 10-2) О НРОД2 (Ка 3 10 х 1013)arrow_forward
- OH- + HCOOH ⇋ H2O + HCOO- Identify base and conjucate acid, and predict which side of the equilibrium is favored? Given: Ka of HCOOH = 1.77 x 10-4arrow_forwardDraw the structures of the conjugate bases of the following acids:arrow_forwardFill in the missing chemical formulae in the tables below. acid + H₂O HCIO₁ H₂CO3 conjugate base П 0 0 base co² OH H₂PO4 conjugate acid 0 П Sarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY