
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
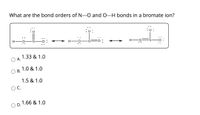
Transcribed Image Text:What are the bond orders of N---O and O---H bonds in a bromate ion?
H -O
1.33 & 1.0
A.
1.0 & 1.0
В.
1.5 & 1.0
C.
1.66 & 1.0
D.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the Lewis structure of CH₃CCH and then choose the appropriate set of hybridization states for the three central atoms. Your answer choice is independent of the orientation of your drawn structure.arrow_forwardThe bond angles marked a, b, and c in the molecule below are about H H :O: H :O: CCCH H 120°, 120°, 90° 109.5°, 120°, 109.5° 120°, 120°, 109.5° O 90°, 90°, 90° 109.5°, 90°, 120° and respectively.arrow_forwardHow many unpaired electrons doe the molecule Liz have? What's the bond order of the diatomic molecule Li2? Is Liz a stable species? Is it paramagnetic or diamagnetic? 2 unpaired electrons; bond order 1; stable; paramagnetic O 2 unpaired electrons; bond order 1; stable; diamagnetic O unpaired electrons; bond order 1; stable; paramagnetic O unpaired electrons; bond order 1; stable; diamagneticarrow_forward
- How many sigma (o) and pi (1) bonds are in the following molecule? Н H-C3 A. 6а, 2п R, 5а. Зп с.6а, 1п D. 5а, 2п Н нarrow_forward11. Draw an octet-rule Lewis structure for Co,2-. State which orbitals or hybrids on C and o overlap to make each valence bond, state each bond type (o or n), and the total bond order between each pair of bonded atoms:arrow_forwardA. Identify which atomic orbitals are used to construct the specified sigma bonds: Bond a is bond b is: bond c 1s1 Identify which orbital is used to construct the a-bond at the marked atom: B. Identify which atomic orbitals are used to construct the specified sigma bonds: a, CH2 HC=C b. H. Bond a is bond b is: bond c is: Identify which orbital is used to construct the T-bond at the marked atom Submit Answer Try Another Version 10 item attempts remainingarrow_forward
- Complete the following table: Lewis structure atoms: bonds between orbitals making each total bond order hybridization atoms: type E valence bond: between two atoms |Н: 1s C and H: o sp?(C) + 1s(H) C and H: 1 C: sp? Cl: sp3 0: sp? :0: || C and Cl: o sp?(C) + sp°(CI) C and Cl: 1 = sp?(C) + sp²(0) = p(C) + p(0) C and O: o C and O: 2 CI C and O: t C: C and C: C and C: C and C: C: H-C=C-H H: C and H: C and C: H: C and H: N: N and I: N and I: N= I: N and I: C: C and Cl: C and Cl: :Cl Cl: C and Cl: C and Cl: Cl: C and Cl: -c=cl: C and Cl: :Z: :U:arrow_forward7.Answer the questions below regarding the following species: N2, O2, O2', O2 based on the molecular orbital diagram. 2p 2p 25 25 press 1100 the Copyright © 2006 Pearson Prentice Hall, Inc. (a) Which species do not contain any unpaired electrons? -- (b) Which has a bond order of 1? (c) Which has a bond order of 3? -- (d) Which has 3 electrons in the T*2p orbital? --- Energyarrow_forwardWhat is the hybridization of the atoms in the molecule shown below? Do not consider any resonance contributors of this molecule.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY