
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
4,5
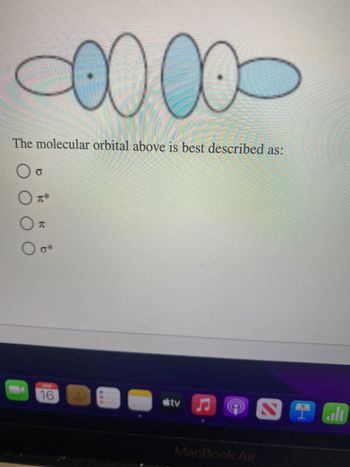
Transcribed Image Text:The molecular orbital above is best described as:
Oo
元
0*
0000
APR
16
tv
MacBook Air
i all

Transcribed Image Text:888
The molecular orbital above is best described as:
O T*
O
T
0*
16
tv
I
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ter answ Aqueo us sulfurous acid (H2S03) was made by dissolving 0.200 L of sulfur dioxide gas at 19°C and 745 mm Hg in water to yield 500.0 mL of solutio n. The acid solution required 17.6 mL of so dium hydroxide solution to reach the titration end point. What was the molarity of the sodium hy droxide solution? M NaOH IID $4 7. COarrow_forwardA buret is used to measure the volume of FeCl3 aqueous solution. The starting volume is recorded to be 5.28 mL. The ending volume is recorded as 17.44 mL. What is the total volume of FeCl3 solution dispensed?arrow_forward2.) Five standardized dilutions of aqueous lead(II) chromate solution were prepared by adding a volume of 0.0010 M PbCrOa(ag) from a buret and then diluting the solution with deionized water to a total volume of 100.0 mL. Use the provided information to determine [CrO42] in each. Hint: enter the decimal before the scientific notation. Mixture Initial Buret Reading (mL) Final Buret Reading (mL) [CrO4?] (M) 5 16.83 42.60 ? x 104arrow_forward
- 7B.arrow_forwardConsider the following reactions:CoO (s) + CO (g) D CO2 (g) + Co (s) Kc(1) = 490.2 CoO (s) + 2 H2 (g) D 2 Co (s) + 2 H2O (g) Kc(2) = 4.5 x 103a. Write the overall equation for the reaction of hydrogen gas and carbon dioxide gas to produce carbon monoxide gas and steam.arrow_forwardA 1.53 g sample of a mixture of FeCl2 and Fe(NO3)2 is treated with excess AgNO3(aq). The precipitate is filtered off, dried and weighed. The dried precipitate weighs 1.92 g. What is the percentage by mass of FeCl2 in the original mixture? Your answer must be accurate to two significant digits. Keep several extra digits in your calculations and round off only at the end. Do not include the percent sign (%) as part of your answer. Molar masses (in g mol−1): N, 14.01 O, 16.00 Cl, 35.45 Fe, 55.85 Ag, 107.9arrow_forward
- Ll.8.arrow_forwardIf 426 mL of 0.204 M HCI solution is needed to neutraize a solution of Ca(OH)2, how many grams of Ca(OH)); must be in the solution? Express the mass in grams to three significant digits.arrow_forwardA student needs to prepare 250 mL of a 0.850 M aqueous solution of sucrose, C12H22O11(aq), which is used frequently in biological experiments.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY