
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
This question was rejected, but this question is part of a practice set and as you can see it is out of zero points. Other practice questions from this set were answered.
Transcribed Image Text:W. Practice 4 - CHEM 1201 X
co Course: 2020 Spring CHEM
b My Questions | bartleby
A https://www.webassign.net/web/Student/Assignment-Responses/randomize?pos=13&dep=22671454&tags=autosave#question361047_13
...
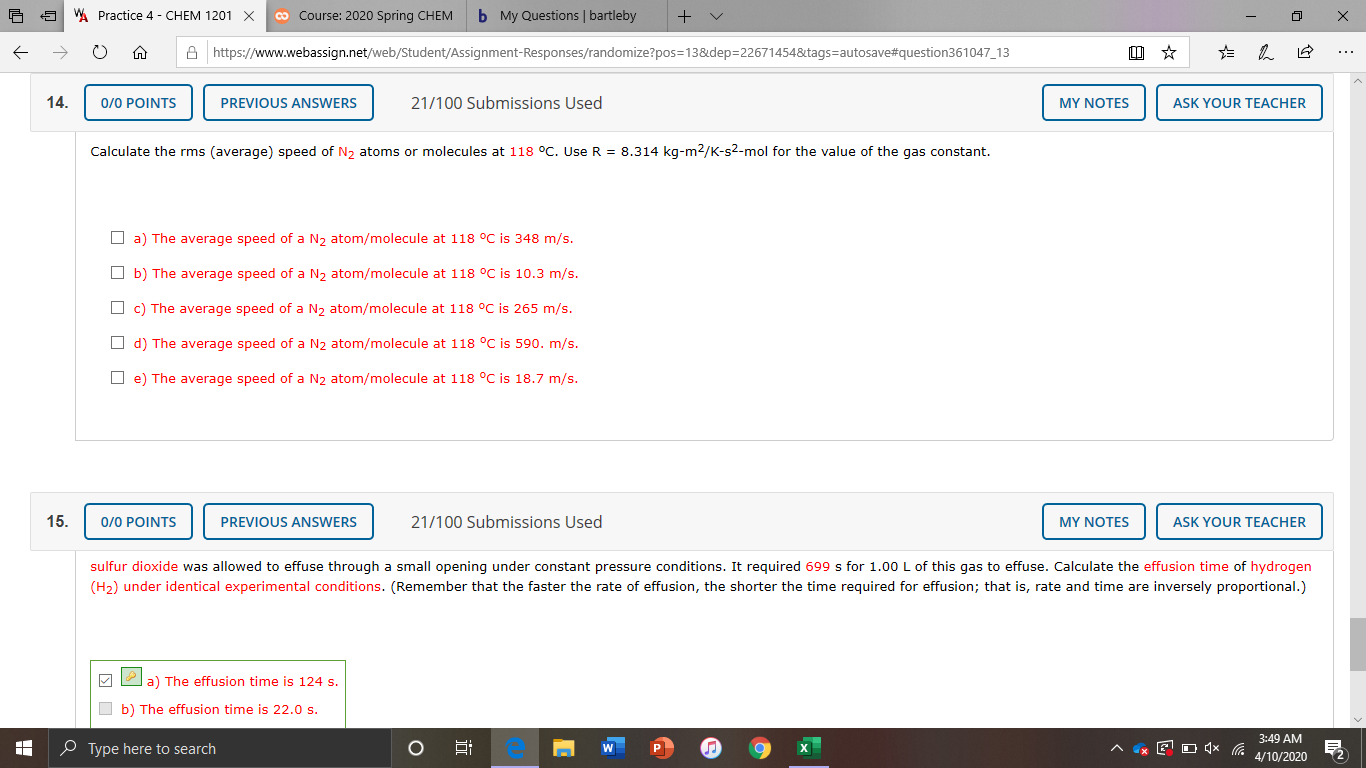
14.
0/0 POINTS
PREVIOUS ANSWERS
21/100 Submissions Used
MY NOTES
ASK YOUR TEACHER
Calculate the rms (average) speed of N, atoms or molecules at 118 °C. Use R = 8.314 kg-m2/K-s2-mol for the value of the gas constant.
O a) The average speed of a N, atom/molecule at 118 °C is 348 m/s.
O b) The average speed of a N, atom/molecule at 118 °C is 10.3 m/s.
O c) The average speed of a N2 atom/molecule at 118 °C is 265 m/s.
O d) The average speed of a N2 atom/molecule at 118 °C is 590. m/s.
O e) The average speed of a N2 atom/molecule at 118 °C is 18.7 m/s.
15.
0/0 POINTS
PREVIOUS ANSWERS
21/100 Submissions Used
MY NOTES
ASK YOUR TEACHER
sulfur dioxide was allowed to effuse through a small opening under constant pressure conditions. It required 699 s for 1.00 L of this gas to effuse. Calculate the effusion time of hydrogen
(H2) under identical experimental conditions. (Remember that the faster the rate of effusion, the shorter the time required for effusion; that is, rate and time are inversely proportional.)
a) The effusion time is 124 s.
b) The effusion time is 22.0 s.
3:49 AM
e Type here to search
4/10/2020
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The solution calls for an exponent. How would I write that?arrow_forwardStep 2: Identify the dimensions of the quantities involved The second step is to identify the dimensions of the quantities involved in the problem. For example, if the problem involves distance, time, and velocity, the dimensions of these quantities would be length, time, and length/time, respectively. Step 3: Check if the units cancel out The third step is to check if the units cancel out. To do this, multiply the quantities together and check if the units cancel out, leaving only the desired unit. For example, if you are trying to find the velocity of an object and you know its distance and time, you can multiply distance by time to get velocity. If the units cancel out, you have a physically meaningful result. Step 4: Check if the result makes sense, to do this compare the units of the result with what you would expect based on the Robles statement. Using the step hints above, answer the question. You do not have to solve. Just imagine that you're teaching a friend how…arrow_forwardWhat is wrong with the following statement? "The results of the experriment do not agree with the theory. something must be wrong with the experiement"arrow_forward
- Explain in reasoning how you would teach him to determine if his dimensional analysis is answers "make sense".arrow_forwardChrome File Edit View History Bookmarks People Tab Window Help Hcc Dashbc x E Buy Es: x G find so x © Periodi x A ALEKS X HUc Chapte x E New m x G conver X 不→ C A www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNsIkr7j8P3jH-IQiHqRdYV_6Ux63SypJXz0Coxvwqgg4JkWI7FgD9QGpr.. O GASES O OC D Jacqueline v Using Avogadro's Law Hydrogen gas and nitrogen gas react to form ammonia gas. What volume of ammonia would be produced by this reaction if 7.5 m of nitrogen were consumed? Also, be sure your answer has a unit symbol, and is rounded to 2 significant digits. 圖 中 ロ alo oloarrow_forward7arrow_forward
- Chrome File Edit View History Bookmarks People Tab Window Help * 30% (4) Sat 2:00 PM Q OE o Chem101 O General Chemistry I (LAB SCI) X + i app.101edu.co EApps MM Gmail YouTube 9 Maps A Translate https://www.carth. 9 Google Chrome isn't your default browser Set as default Question 7 of 7 Submit Using the equations 2 Sr(s) + O2 (g) → 2 Sro (s) AH° = -1184 kJ/mol CO2 (g) → C (s) + O2 (g) AH° = 394 kJ/mol kJ/mol Determine the enthalpy for the reaction C(s) + 2 SrO(s) → CO2 (g) + 2 Sr(s). 1 2 6 C 03.0 Se VHFORMA The MO -Standa 8 AH ma +/- x 100 8T國山電@ O etv Oct 24 MacBook Air 80 888 SC FS F6 F3 F2 %23 2$ & delete 3 5 8. W E R Y U H J к K ret F V M 4-arrow_forwardI have calculated this problem various times, but every time I input the answer into the assignment it says it's wrong, I can't find the issue. What could it be?arrow_forwardIf this piece of blue litmus paper is placed in that acid solution,then this piece of blue litmus paper will turn red.which of the following is true of this conditional statement: A. The antecedent is "this piece of blue litmus paper will turn red." B. This statement is false. C. This statement cannot serve as a premise in an argument. D. This statement cannot be the conclusion of an argument.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY