
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN: 9781305580343
Author: Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Show work. don't give Ai generated solution
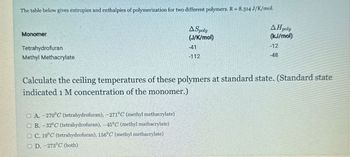
Transcribed Image Text:The table below gives entropies and enthalpies of polymerization for two different polymers. R= 8.314 J/K/mol.
Monomer
Tetrahydrofuran
Methyl Methacrylate
A Spoly
(J/K/mol)
-41
-112
AH poly
(kJ/mol)
-12
-48
Calculate the ceiling temperatures of these polymers at standard state. (Standard state
indicated 1 M concentration of the monomer.)
A. -270°C (tetrahydrofuran), -271°C (methyl methacrylate)
B. 32°C (tetrahydrofuran), -45°C (methyl mathacrylate)
C. 19°C (tetrahydrofuran), 156°C (methyl methacrylate)
D. -273°C (both)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Differential Thermal Analysis (DTA) and Differential Scanning Calorimetry (DSC) are used to support the information obtained from Thermogravimetric Analysis (TGA) of polymers. Explain.arrow_forward2. The Tg of poly(vinyl acetate) is listed as 29°C. If 5 mol % of divinyl benzene is copolymerized in the polymer during polymerization, what is the new glass transition temperature?arrow_forwardAssume the energy of hydrogen bonds per base pair to be 5.86 kJ•mol-1. Given two complementary strands of DNA containing 145 base pairs each, calculate the ratio of two separate strands to hydrogen-bonded double helix in solution at 319 K. ratio = .44arrow_forward
- A characteristic of the stoichiometry of condensation (step-growth) polymerization reactions is that small molecules, like H2O, HCl or others, are released as reaction by-products. In a commercial polymerization reactor, what might be some process implications of the release of small molecules like H2O or HCl?arrow_forward18)arrow_forwardMARCA 108. Using the following data, calculate the standard heat of forma- tion of ICI(g) in kJ/mol: → 2C1(g) 21(g) Cl,(3) AH° 242.3 kJ AH° 151.0 kJ ICI(g) → I(g) + C1(g) AH° = 211.3 kJ I,(s) AH° = 62.8 kJ >arrow_forward
- Phase change properties of pure substances Cu C6H12 C (C6H5CH₂)₂0 (CH3CH₂)20 CH3CH₂OH (CH₂OH)2 NH₂COH Au C C6H14 H₂ copper cyclohexane diamond dibenzyl ether diethyl ether ethanol ethylene glycol formamide gold graphite hexane hydrogen 1084.62 6.7 4440 1.8 -116.22 -114.14 -13.0 2.57 1064.18 4489 -95.27 -259.16 2560 80.7 298 34.4 78.24 197.5 217 2836 3825 68.72 -252.879 0.385 1.841 0.51 2.369 2.438 2.394 2.389 0.129 0.709 2.27 14.304 13.26 2.68 -- 7.19 4.931 9.96 8.44 12.55 117.4 13.08 0.12 29.97 45.6 26.52 38.56 50.5 60.2 324 28.85 0.9 280.3 193.7 242 446 -- 234.4 -240.212 40.2 35.9 61.7 80 29.9 12.69arrow_forwardIn a free - radical polymerization of styrene in solution at 600C, carbon tetrabromide was used as a chain transfer agent. The initial concentrations of styrene and CBr4 were 1 mol/L and 0.01 mol/L, respectively. In 1 h, these concentrations dropped to 0.85 mol/L and 0.007 mol/L, respectively. What is the chain transfer constant CS for styrene/CBr4? (Neglect chain transfer to monomer, initiator, and solvent.)arrow_forwardThe heat of fusion of polyethylene is approximately 7.7 kJ/mol, and the corresponding entropy is 19 J mol-1 K-1. Use these data to estimate the melting point of polyethylene.arrow_forward
- Draw a log E – temperature plot for a linear, amorphous polymer.(a) Indicate the position and name the five regions of viscoelastic behavior.(b) How is the curve changed if the polymer is semicrystalline?(c) How is it changed if the polymer is cross-linked?(d) How is the curve changed if the molecular weight is increased significantly?(e) How is it changed if the experiment is run at higher frequency—that is, if measurements are made after 10 Hz rather than 1 Hzarrow_forwardWhy is the average molar mass of a polymer sample dif-ferent from the molar mass of an individual chain?arrow_forward9. Consider the two polymers Nylon 6,10, with the degree of polymerization of 100 and poly(ethyl methacrylate) with the degree of polymerization of 1200. (a) Write chemical structures of the polymers. (b) What polymer will have a higher value of real end-to-end distance in bulk? Assume that the values of characteristic ratio and bond length are the same for these polymers. 1/2 The equation for the end-to-end distance: (²) 11² = α (nCN) 1/21arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax