
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Consider 2.00 moles of an ideal gas that are taken from state A (PA = 2.00 atm, vA = 10.0 L) to state B (PB = 1.00 attn, VB = 30.0 L) by two different pathways:
These pathways are summarized on the following graph of P versus V:
Calculate the work (in units of J) associated with two pathways. Is work a state function? Explain.
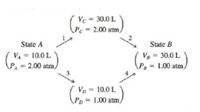
Transcribed Image Text:Ve = 30.0L
Pc = 2.00 atm,
State A
State B
VA - 10.0 L
(P, = 2.00 atm/
V = 30.0 L
\P, = 1.00 atm,
Vo = 10.0L
Pp- 1.00 atm
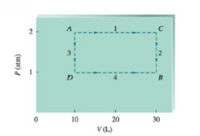
Transcribed Image Text:10
20
30
V(L)
(uw)d
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A cylinder with a piston contains 0.338 mol of ideal gas at 5.10 x 105 Pa and 349 K. Consider these paths: (1) The gas expands isobarically to 3.6 of its original volume. (2) It is then compressed isothermally back to its original volume. (3) It is cooled isochorically to its original pressure in the end. a. Show the series of processes on a pV-diagram. b. Compute the temperature during the isothermal compression; c. Compute the maximum pressure; d. Compute the total work done by the piston on the gas during the series of processes.arrow_forwardSix thermodynamic states of the same monatomic ideal gas sample are represented in the figure. (Figure 1) Part C Rank the states on the basis of the internal energy of the gas sample. Rank from largest to smallest. To rank items as equivalent, overlap them. • View Available Hint(s) Reset Help E F В A Figure 1 of 1 largest smallest F The correct ranking cannot be determined. C E Submit Aarrow_forwardNeeds Complete typed solution with 100 % accuracy.arrow_forward
- n moles of a diatomic ideal gas go through cycle a → b → c → d → a as shown in the figure. Processes ab and cd are isothermal and occur at temperatures TH and TC, respectively. 1) Calculate the work Wab done by the gas during the process a → b in terms of the variables in the problem. 2) Calculate the work Wbc done by the gas during the process b → c. 3) Calculate the total work W done in the entire cycle in terms of the variables in the problem. 4) Calculate the total heat Q flowing into the gas in a complete cycle.arrow_forwardConsider a process that uses n moles of a monatomic ideal gas operating through a Carnot cycle. The initial temperature and pressure of the gas are T1 and P1, respectively. Consider steps 1 → 2, 2 → 3, 3 → 4, and 4 → 1. In the adiabatic heating, the temperature of the gas is doubled. Write an expression for the volume V3 after this step in terms of V1. Write an expression for P3 in terms of n, R, T3 and V3.arrow_forwardAn aircraft engine takes in 8900 JJ of heat and discards 6700 JJ each cycle. Part A. What is the mechanical work output of the engine during one cycle? Part B. What is the thermal efficiency of the engine? Express your answer as a percentage.arrow_forward
- You have an ideal gas that expands from 0.50 to 4.0 L at a constant temperature of 300K. The gas does 250 J of work. How many moles of gas are there?arrow_forwardHow do I go about this question?arrow_forwardA Carnot engine operates with an efficiency of 30%. The temperature of the cold reservoir remains at 10°C. a. Calculate the temperature of the hot reservoir. TH = %3D b. By how much does the temperature in the hot reservoir need to be raised so that the efficiency of the engine becomes 50%. Express your answer in degrees Celsius.arrow_forward
- The heat of melting of ice at 1 atmosphere pressure and 0°C is 1.4363 kcal/mol. The volume of ice is 0.0196 liter and the volume of water is 0.018 liter. If 1 mole of ice is melted under these conditions, what will be a. The work done in cal? b. The change in internal energy in cal? c. The change in entropy in cal? Note Note that 1 literxatmosphere is equal to 101.325 joule. Sol": given Heat of melting ice at 1 atm pressure = 1.4363 kca kcal/ 2 = 1.4363 kcal/mole mole volume of ice = 0·0196 lts " water = 0.018 lts @ work done is define as w=pdv » w = P(V₁ hot W = 1 (0·018-00196) W = 0·0016 Cal Pay Kat Ansarrow_forwardSpontaneous processes do not exists 2. Which of the following mechanical processes are not reversible, in the 2 sense that energy converted from one form to another cannot be completely converted from the second form back to the first form? * O A ball is tossed upward, gaining height. A moving block slides to a stop because of friction. O A moving cart is caught by a spring, stretching the spring.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON