
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
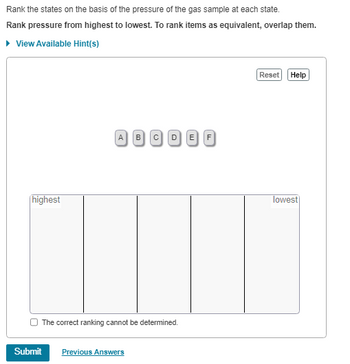
Transcribed Image Text:Rank the states on the basis of the pressure of the gas sample at each state.
Rank pressure from highest to lowest. To rank items as equivalent, overlap them.
▸ View Available Hint(s)
highest
A39099
(c)
The correct ranking cannot be determined.
Submit
Previous Answers
Reset Help
lowest
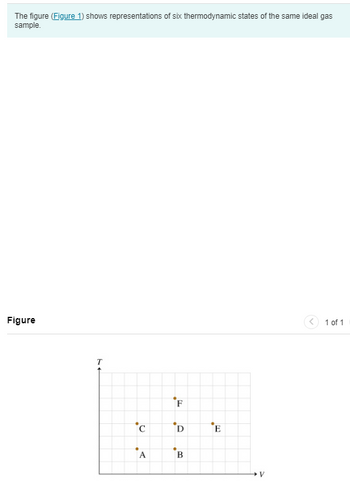
Transcribed Image Text:The figure (Figure 1) shows representations of six thermodynamic states of the same ideal gas
sample.
Figure
T
C
A
F
D
B
E
V
1 of 1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Use the worked example above to help you solve this problem. A cylinder contains 2.15 mol of helium gas at 18.5°C. Assume that the helium behaves like an ideal gas. (a) Find the total internal energy of the system. J(b) What is the average kinetic energy per molecule? J(c) How much energy would have to be added to the system to double the rms speed? The molar mass of helium is 4.00 10-3 kg/mol. Jarrow_forwardA container holds a sample of ideal gas in thermal equilibrium, as shown in the figure. (Figure 1) One end of the container is sealed with a piston whose head is perfectly free to move, unless it is locked in place. The walls of the container readily allow the transfer of energy via heat, unless the piston is insulated from its surroundings. Refer to the pV diagram presented to answer the questions below. (Figure 2) In each case, the piston head is initially unlocked and the gas is in equilibrium at the pressure and volume indicated by point 0 on the diagram. Starting from equilibrium at point 0, what point on the pV diagram will describe the ideal gas after the following process? "Lock the piston head in place, and hold the container above a very hot flame." Starting from equilibrium at point 0, what point on the pV diagram will describe the ideal gas after the following process? "Immerse the container into a large water bath at the same temperature, and very slowly push the piston…arrow_forwardQUESTION 16 The temperature at state A is 20.0°C, that is 293 K. During the last test, you have found the temperature at state D is 73.0 K and n = 164 moles for this monatomic ideal gas. What is the change in thermal energy for process A to D, in MJ (MegaJoules)? Your answer needs to have 2 significant figures, including the negative sign in your answer if needed. Do not include the positive sign if the answer is positive. No unit is needed in your answer, it is already given in the question statement. p (atm) 5 4 3 2 1 0 A D 1 2 3 4 B 5 → V (m³)arrow_forward
- Problem 1: Describe a situation in which the entropy of a container of gas is constant. In other words, come up with your own problem where the answer is that AS = 0.arrow_forwardProblem 5 Consider a substance that has a melting temperature of T= 159 K at p=1 atm and a melting enthalpy of AHm = 4900 J/mol. Determine the entropy of melting the substance.arrow_forwardFor the diagram shown below, if an ideal gas transition from point A to point D, then: the Temperature of the gas sample will____? the Pressure of the gas sample will______?arrow_forward
- So each four dot shows four different states for an ideal gas. P is the pressure and the other one is the density of the gas. Is temperature of state 1 less or greater than the state 2? Can you please explain why?arrow_forwardLook at the P-V diagram below (Diagram 1). Calculate the work done by the gas for the paths A, B and C. Assume that in Diagram 1, P1 = 1 atm, P2 = 4 atm, V1 = 5 L, V2 = 15 L. a) WA = 1013 J, WB = 0, WC = -2533 J b) WA = 0.01 J, WB = 0, WC = -0.025 J c) WA = 2533 J, WB = 0, WC = -1013 J Calculate the work done by the gas for the path AB in Diagram 2. Use the data: P1 = 1 atm, P2 = 4 atm, V1 = 5 L, V2 = 20 L. (Path AB is an "isothermal" which means the temperature T is constant on this path). a) 0.012 J b) 1220 J c) 0.0278 J d) 2809 Jarrow_forwardA container is filled with an ideal diatomic gas to a pressure and volume of P1 and V1, respectively. The gas is then warmed in a two-step process that increases the pressure by a factor of five and the volume by a factor of four. Determine the amount of energy transferred to the gas by heat if the first step is carried out at constant volume and the second step at constant pressure. (Use any variable or symbol stated above as necessary.)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON