
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
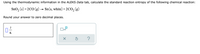
Transcribed Image Text:Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction entropy of the following chemical reaction:
SnO₂ (s) +2CO (g) → Sn(s, white) +2CO₂ (g)
Round your answer to zero decimal places.
x10
X
0-+-+-
K
Ś ?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- es) Qualitatively predicting reaction entropy For each chemical reaction listed in the first column of the table below, predict the sign (positive or negative) of the reaction entropy AS If it's not possible to decide with the information given, check the "not enough information" button in the last column. Note for advanced students: Assume the temperature remains constant. Assume all gases and solutions are ideal. reaction N₂(g) + 3H₂(g) → 2NH, (e) 4Fe()+30.)- 2Fe₂O, (s) N. (3), (g) 2NH, (g) sign of reaction entropy 04 ron reaction <<0 50 not enough information. 45x 250 <<0 not enough information. 450 50 not enough information. Using the general properties of Gibbs free energy The standard reaction free energy AG" --835. kJ for this reaction: 2 Al(s) + Fe₂O3(s)-Al₂O3(s) + 2 Fe(s) Use this information to complete the table below. Round each of your answers to the nearest kJ. BALAKEN F04 AGO Focusarrow_forwardFor each chemical reaction listed in the first column of the table below, predict the sign (positive or negative) of the reaction entropy AS If it's not possible to decide with the information given, check the "not enough information" rxn' button in the last column. Note for advanced students: Assume the temperature remains constant. Assume all gases and solutions are ideal. reaction 4Fe(s) + 30₂ (g) 2 Fe₂O3 (s) Ag Cl (s) → Ag+ (aq) + Cl¯(aq) 2NH3(g) N₂(g) + 3H₂(g) sign of reaction entropy AS rxn AS > 0 rxn not enough information. 0 rxn not enough information. AS 0 not enough information.arrow_forwardUsing the thermodynamic information in the ALEKS Data tab, calculate the standard reaction entropy of the following chemical reaction: P4010 (s) + 6H₂O (1)→ 4H₂PO4(s) Round your answer to zero decimal places. x10 0 -/-/ K X ? Śarrow_forward
- Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction entropy of the following chemical reaction: C,H; (g) + 50, (g) - 3CO, g) + 4H,0 (1) Round your answer to zero decimal places. J x10 Karrow_forwardCan you help me with this?arrow_forwardIp acellus_engine.html?ClassID=D1653724337# Calculate the entropy of the phase change of uranium hexafluoride from gas to solid. UF6(g) → UF6(s) Substance S° (J/mol-K) UF (s) UF (g) ASiep = [ ? ] J/mol - K 228 380 Enter either a + or - sign AND the magnitude in your answer. Enterarrow_forward
- Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction entropy of the following chemical reaction: CH,OH (g)+CO (g) → HCH,CO₂ (1) Round your answer to zero decimal places. -0 K Xarrow_forward1) Predict the sign (+ or -) of the entropy for the system and justify your answer. H2 (g) + ½ O2 (g) → H2O (l) 2) Calculate the entropy change for each of the above using your data tables. H2 (g) + ½ O2 (g) → H2O (l) ΔS° =? Note: Please answer number 1 and 2 Thank you.arrow_forwardUsing the thermodynamic information in the ALEKS Data tab, calculate the standard reaction entropy of the following chemical reaction: Al,03 (s)+ 3H, (g) → 2Al (s) + 3H,O (g) Round your answer to zero decimal places. x10 Karrow_forward
- Using the thermodynamic information in the ALEKS Data tab, calculate the standard reaction entropy of the following chemical reaction: SnO, (s) +2CO (g) → Sn(s, white)+2CO, (g) Round your answer to zero decimal places. Ox10 Karrow_forwardUsing the thermodynamic information in the ALEKS Data tab, calculate the standard reaction free energy of the following chemical reaction: C (s) +2Cl, (g) → CCI, (g) Round your answer to zero decimal places. | kJarrow_forwardFor each chemical reaction listed in the first column of the table below, predict the sign (positive or negative) of the reaction entropy ASxn. If it's not possible to decide with the information given, check the "not enough information" button in the last column. Note for advanced students: Assume the temperature remains constant. Assume all gases and solutions are ideal. reaction + Ag (aq) + Cl (aq) Ag Cl (s) C₂H₂(g) + 2H₂(g) → C₂H₂(g) 6 MgCl₂ (s) + H₂O(1) MgO (s) + 2H C1 (g) sign of reaction entropy AS 0 rxn not enough information. AS 0 rxn not enough information. AS 0 not enough information. Xarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY