
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:es)
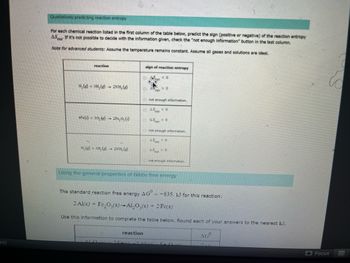
Qualitatively predicting reaction entropy
For each chemical reaction listed in the first column of the table below, predict the sign (positive or negative) of the reaction entropy
AS If it's not possible to decide with the information given, check the "not enough information" button in the last column.
Note for advanced students: Assume the temperature remains constant. Assume all gases and solutions are ideal.
reaction
N₂(g) + 3H₂(g) →
2NH, (e)
4Fe()+30.)- 2Fe₂O, (s)
N. (3), (g) 2NH, (g)
sign of reaction entropy
04
ron
reaction
<<0
50
not enough information.
45x
250
<<0
not enough information.
450
50
not enough information.
Using the general properties of Gibbs free energy
The standard reaction free energy AG" --835. kJ for this reaction:
2 Al(s) + Fe₂O3(s)-Al₂O3(s) + 2 Fe(s)
Use this information to complete the table below. Round each of your answers to the nearest kJ.
BALAKEN F04
AGO
Focus
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For one day, keep a log of all the activities you undertake that consume Gibbs free energy. Distinguish betweenGibbs free energy provided by nutrient metabolism andthat provided by other energy resources.arrow_forwardExplain how the entropy of the universe increases when an aluminum metal can is made from aluminum ore. Thefirst step is to extract the ore, which is primarily a formof A12O3, from the ground. After it is purified by freeingit from oxides of silicon and iron, aluminum oxide ischanged to the metal by an input of electrical energy. 2Al2O3(s)electricalenergy4Al(s)+3O2(g)arrow_forwardChemists and engineers who design nuclear power plants have to worry about high-temperature reactions because it is possible for water to decompose. (a) Under what conditions does this reaction occur spontaneously? 2H2O(g) 2H2(g) + O2(g) (b) Under conditions where the decomposition of water is spontaneous, do nuclear engineers have to worry about an oxygen/hydrogen explosion? Justify your answer.arrow_forward
- Someone once suggested that it is impossible to unscramble a scrambled egg. Describe an unscrambled and a scrambled egg in terms of the concept of entropy.arrow_forwardWhat is entropy? Why is entropy important?arrow_forwardWhen (if ever) are high temperatures unfavorable to a reaction thermodynamically?arrow_forward
- Explain why absolute entropies can be measured.arrow_forwardWhat is the sign of the standard Gibbs free-energy change at low temperatures and at high temperatures for the synthesis of ammonia? 3H2(g) + N2(g) 2NH3(g)arrow_forwardEntropy has been described as times arrow. Interpret this view of entropy.arrow_forward
- Which contains greater entropy, a quantity of frozen benzene or the same quantity of liquid benzene at the same temperature? Explain in terms of the dispersal of energy in the substance.arrow_forwardA key component in many chemical engineering designs is the separation of mixtures of chemicals. (a) What happens to the entropy of the system when a chemical mixture is separated? (b) Are designs for chemical separation more likely to rely on spontaneous or nonspontaneous processes?arrow_forwardThe standard molar entropy of methanol vapor, CH3OH(g), is 239.8 J K1 mol-1. (a) Calculate the entropy change for the vaporization of 1 mol methanol (use data from Table 16.1 or Appendix J). (b) Calculate the enthalpy of vaporization of methanol, assuming that rS doesnt depend on temperature and taking the boiling point of methanol to be 64.6C.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning