
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please draw it for me
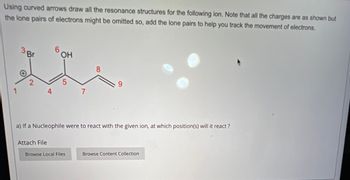
Transcribed Image Text:Using curved arrows draw all the resonance structures for the following ion. Note that all the charges are as shown but
the lone pairs of electrons might be omitted so, add the lone pairs to help you track the movement of electrons.
Br
6
HO
OH
2
5
1
4
7
8
9
a) If a Nucleophile were to react with the given ion, at which position(s) will it react?
Attach File
Browse Local Files
Browse Content Collection
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- for part b)why do we write AlCl3 at top of the arrow and AlCl4 at the bottom of arrow??is it any connection between the two?does it means that AlCl 4 need to be present in order to make AlCl3 reacts to the molecule and make the reaction to process?? 2. why when AlCl3 added,Cl was removed from the ring?what princeiple theory is that ? 2. when SO3 is added ,SO3 attached to the ring,why ?what principle is behind this phenamonane 3.Just confused about why AlCl3 removes Cl from the ring,while HSO3 donate SO3 to the ring. Is it Something about nucleohilic or eletrciohillic?arrow_forwardnaming a chemical compound. I'm currently learning how to name alkanes and alkyl halides. Im confused about the compound ethanal, or acetaldehyde. The second carbon has a double bond on its hydrogen, so I'm wondering why the infix is "an" instead of "en". Frpm the table of infixes, it says "an" is for single bonds. Can someone help me understandarrow_forwardThe organic acid propanoic acid will react with the organic base cyclohexylamine. *O* H . All hydrogen atoms are implied, except: ● a Draw the structures of the products of this neutralization reaction. H H o Hydrogen atoms not attached to carbon atoms o Hydrogen atoms involved in the mechanism **** Apply formal charges where appropriate. Assign lone pairs and radical electrons where appropriate. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. . Separate multiple products using the + sign from the drop-down menu. ● If needed, use the "starting points" menu to revert to the original molecule(s) shown. ? Sn [F]arrow_forward
- Draw all missing reactants and/or products in the appropriate boxes by placing atoms on the canvas and connecting them with bonds. Add charges where needed. Electron flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created.arrow_forward3. Use curved arrows to show electron movement in the reactant side and draw the product/s of the Lewis (nucleophile-electrophile) reaction. Draw in all lone pairs and charges where appropriate. acid-base -co +arrow_forward8)) Fill in the missing structures, the reactive intermediate and the final product. Then descr how the reactant and final product could be differentiated by IR. reactant product 1. NaNH2. 2. EtBrarrow_forward
- Draw structural formulas for organic products A and B in the window below. Br pentane Cul A B. • Draw only products having the organic portion of the original alkyl halide. • Draw carbon-lithium bonds using the single bond tool. If a structure has a copper-lithium bond, do not draw the lithium. Separate products from different steps using thesign from the drop-down menu.arrow_forward[Review Topics] [Refer Draw structural formulas for the major organic product(s) of the reaction s CN AICI3 + (CH3)2CHCI • • • . . You do not have to consider stereochemistry. If no reaction occurs, draw the organic starting material. Remember to include all of the formal charges on the atoms of any Draw one structure per sketcher. Add additional sketchers using th corner. Separate multiple products using the + sign from the drop-down m 26224 N▾ ? [ ChemDoodle removearrow_forwardPls help ASAP.arrow_forward
- 2) Draw the Lewis structures first and then complete the following acid-base reaction by using appropriate arrows. Acid and base should be labeled. CH3CHBRCOOH + NaHarrow_forwardDd.80.arrow_forwardComplete the equation for the reaction between the following Lewis acid-base pair. Use curved arrows to show the flow of electrons in the reaction and draw the product. Assign lone pairs and radical electrons where appropriate. Apply formal charges where appropriate. • Draw the appropriate electron-flow arrows. • Use the "starting points" menu to revert to the original molecule(s) shown. • Omit + signs between structures. ● / CH3 1- H₂C-C CH3 H در St ? ChemDoodleⓇarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning