
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:O
|||
rosoft C
W
Microsoft
Microsoft
6.52.210...
esc
OSTATES OF MATTER
Understanding the connection between vapor pressure, boiling...
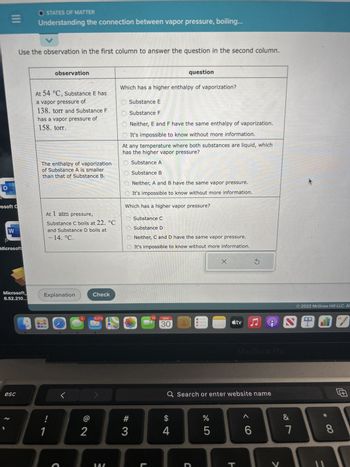
Use the observation in the first column to answer the question in the second column.
V
observation
At 54 °C, Substance E has
a vapor pressure of
138. torr and Substance F
has a vapor pressure of
158. torr.
The enthalpy of vaporization
of Substance A is smaller
than that of Substance B.
At 1 atm pressure,
Substance C boils at 22. °C
and Substance D boils at
- 14. °C.
Explanation
!
1
5
2
Check
9,073
280
Which has a higher enthalpy of vaporization?
Substance E
Substance F
Neither, E and F have the same enthalpy of vaporization.
It's impossible to know without more information.
At any temperature where both substances are liquid, which
has the higher vapor pressure?
Substance A
Substance B
Neither, A and B have the same vapor pressure.
It's impossible to know without more information.
Which has a higher vapor pressure?
Substance C
O Substance D
Neither, C and D have the same vapor pressure.
It's impossible to know without more information.
#3
question
L
14
NOV
30
$
4
C
%
Q Search or enter website name
LO
X
5
stv
V♫
H
MacBook Pro
< C
6
SO
&
O 2022 McGraw Hill LLC. AL
7
*
8
7
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- O Use the observation in the first column to answer the question in the second column. osoft C W W Microsoft Microsoft 6.52.210... esc At 45 °C, Substance E has a vapor pressure of 146. torr and Substance F has a vapor pressure of 116. torr. observation At 1 atm pressure, Substance A boils at -28. °C and Substance B 56. °C. boils at The enthalpy of vaporization of Substance C is smaller than that of Substance D. ! - Explanation 1 Q S 2 Check 8,976 W 280 Which has a higher enthalpy of vaporization? Substance E Substance F Neither, E and F have the same enthalpy of vaporization. It's impossible to know without more information. Which has a higher vapor pressure? Substance A Substance B Neither, A and B have the same vapor pressure. It's impossible to know without more information. Which has the higher boiling point? Substance C Substance D Neither, C and D have the same boiling point. It's impossible to know without more information. #3 17 E question DEC 10 Pag $ 4 R 27 20 % X tv♫♫ T Seg…arrow_forwardThe heat of vaporization of water is 40.66 kJ/mol. How much heat is absorbed when 3.40 g of water boils at atmospheric pressure? kJ heat:arrow_forwardUse the observation in the first column to answer the question in the second column. observation At 1 atm pressure, Substance E boils at 11. °C and Substance F boils at 30. °C. At 37 °C, Substance C has a vapor pressure of 89. torr and Substance D has a vapor pressure of 79. torr. The enthalpy of vaporization of Substance A is smaller than that of Substance B. question Which has a higher vapor pressure? Substance E Substance F Neither, E and F have the same vapor pressure. It's impossible to know without more information. Which has a higher enthalpy of vaporization? Substance C Substance D Neither, C and D have the same enthalpy of vaporization. It's impossible to know without more information. At any temperature where both substances are liquid, which has the higher vapor pressure? Substance A Substance B Neither, A and B have the same vapor pressure. It's impossible to know without more information. X Śarrow_forward
- Use the observation in the first column to answer the question in the second column. observation The enthalpy of vaporization of Substance C is bigger than that of Substance D. At 1 atm pressure, Substance A boils at 117. °C and Substance B boils at 82. °C. At -2 °C, Substance E has a vapor pressure of 109. torr and Substance F has a vapor pressure of 59. torr. question At any temperature where both substances are liquid, which has the higher vapor pressure? Substance C Substance D Neither, C and D have the same vapor pressure. It's impossible to know without more information. Which has a higher enthalpy of vaporization? Substance A Substance B Neither, A and B have the same enthalpy of vaporization. It's impossible to know without more information. Which has a higher enthalpy of vaporization? Substance E Substance F Neither, E and F have the same enthalpy of vaporization. It's impossible to know without more information. X Sarrow_forwardTry Again Your answer is incorrect. The enthalpy of vaporization of Substance X is 23.0 Round your answer to 2 significant digits. 0.32 atm x10 X kJ and its normal boiling point is 10. °C. Calculate the vapor pressure of Xat - 18. °C. mol Sarrow_forwardThe enthalpy of vaporization of Substance X is greater than that of Substance Y. Which of the following statements is true? O At any temperature where both substances are liquid, substance X will have the higher vapor pressure O At 1 atm pressure, substance Y will have the higher boiling point O At any temperature where both substances are liquid, substance Y will have the lower vapor pressure Substance Y would have IMF's with a greater magnitude than substance X O At 1 atm pressure, substance X will have the higher boiling pointarrow_forward
- Use the observation in the first column to answer the question in the second column. observation The enthalpy of vaporization of Substance E is bigger than that of Substance F. At 36 °C, Substance C has a vapor pressure of 111. torr and Substance D has a vapor pressure of 91. torr. At 1 atm pressure, Substance A boils at 36. °C and Substance B boils at 52. °C. question Which has the higher boiling point? Substance E Substance F Neither, E and F have the same boiling point. It's impossible to know without more information. Which has a higher boiling point? Substance C Substance D Neither, C and D have the same boiling point. It's impossible to know without more information. Which has a higher enthalpy of vaporization? Substance A Substance B Neither, A and B have the same enthalpy of vaporization. It's impossible to know without more information.arrow_forward3. A substance has a heat of vaporization of 28.90 kJ/mol. At -21 °C it has a vapor pressure of 137 mmHg. What is the temperature in °C when the vapor pressure is 14 mmHg? Round to the nearest whole number.arrow_forwardUse the observation in the first column to answer the question in the second column. observation question Which has the higher boiling point? O Substance E The enthalpy of vaporization of Substance E is smaller O Substance F than that of Substance F. Neither, E and F have the same boiling point. It's impossible to know without more information. Which has a higher boiling point? At 73 °C, Substance A has a O Substance A vapor pressure of 95. torr and Substance B has a vapor O Substance B pressure of 125. torr. O Neither, A and B have the same boiling point. O It's impossible to know without more information. Which has a higher vapor pressure? At 1 atm pressure, O Substance C Substance C boils at 14. °C and Substance D boils at O Substance D 37. °C. O Neither, C and D have the same vapor pressure. O It's impossible to know without more information. Continue MacBook Pro Search or tyne UPIarrow_forward
- Use the observation in the first column to answer the question in the second column. observation question Which has the higher boiling point? O Substance E The enthalpy of vaporization of Substance E is smaller Substance F than that of Substance F. Neither, E and F have the same boiling point. It's impossible to know without more information. Which has a higher enthalpy of vaporization? At 36 °C, Substance C has a Substance C vapor pressure of 140. tor and Substance D has a vapor Substance D pressure of 130. torr. Neither, C and D have the same enthalpy of vaporization. It's impossible to know without more information. Which has a higher enthalpy of vaporization? At 1 atm pressure, Substance A Substance A boils at -12. °C and Substance B Substance B boils at 1. °C. Neither, A and B have the same enthalpy of vaporization. It's impossible to know without more information.arrow_forwardPlease help me solve the given question, explain and make sure everything is correct 1000% thanksarrow_forwardSubmit correct and complete solutions. Provide step-by-step detailed explanations.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY