
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
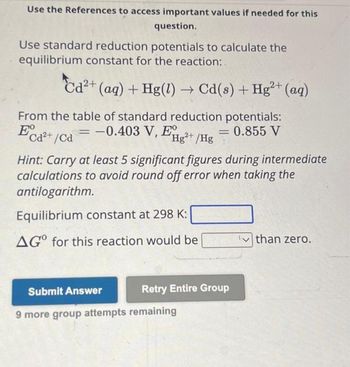
Transcribed Image Text:Use the References to access important values if needed for this
question.
Use standard reduction potentials to calculate the
equilibrium constant for the reaction:
2+
Cd²+ (aq) + Hg(1)→ Cd(s) + Hg²+ (aq)
From the table of standard reduction potentials:
E
= -0.403 V, EHg²+ /Hg
= 0.855 V
2+
Cd²+/Cd
Hint: Carry at least 5 significant figures during intermediate
calculations to avoid round off error when taking the
antilogarithm.
Equilibrium constant at 298 K:
AGO for this reaction would be
Submit Answer
Retry Entire Group
9 more group attempts remaining
than zero.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the cell shown, the measured cell potential, Ecell, is -0.3651 V at 25 °C. Pt(s) | H₂(g, 0.797 atm) | H+ (aq, ? M) || Cd²+ (aq, 1.00 M) | Cd(s) The balanced reduction half-reactions for the cell, and their respective standard reduction potential values, Eº, are 2 H+ (aq) + 2 e- → H₂(g) Cd²+ (aq) + 2e → Cd(s) Calculate the H+ concentration. [H+] = x10 TOOLS - Eº = 0.00 V Eº = -0.403 V Marrow_forwardPlease fill in the columns in table 2, given the answers in table 1. Please point out any errors in table 1 Givens: Potential for any half reaction involving silver is 0.00 Varrow_forwardedit: Calculate the cell potential for the following galvanic cell at 25°C: Al(s) Al3+ (0.0632 M)|| Au3+ (0.255 M)|Au(s)arrow_forward
- Balance the following reaction in acidic solution. Fe?+ (ag) + MnO, (aq) → Fe+ (ag) + Mn²+(aq) Fill in the coefficients and substances for the balanced overall equation. Note: Do NOT leave any coeffient fields empty. If the coefficient is 1, choose "1" as opposed to leaving it blank. Fe2+ + MnO,¯ + 4 Fe3+ + Mn2+ +arrow_forwardFor the cell shown, the measured cell potential, Ecell, is -0.3619 V at 25 °C. Pt(s) | H₂(g, 0.721 atm) | H¹ (aq, ? M) || Cd²+ (aq, 1.00 M) | Cd(s) The balanced reduction half-reactions for the cell, and their respective standard reduction potential values, Eº, are 2 H+ (aq) + 2 e H₂(g) Eº = 0.00 V Cd²+ (aq) + 2e → Cd(s) E° = -0.403 V Calculate the H+ concentration. [H+] = TOOLS x10 Marrow_forwardGive only typing answer with explanation and conclusionarrow_forward
- Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy for the following redox reaction. Round your answer to 3 significant digits. The final units should be in kjarrow_forwardUsing the following standard reduction potentials: Ag+ (aq) + e- ⇌ Ag (s) E o = +0.80 V Pb2+ (aq) + 2e - ⇌ Pb (s) Eo = -0.13 V i. Write a balanced redox reaction that represents the overall cell reaction for the feasible galvanic cell. ii. Calculate the standard cell potential. iii. Draw a fully labelled galvanic cell for this redox system. Your labels should show (but not limited to): the electron and ion flow; the anode and cathode, the half cells where oxidation and reduction take place. B. Describe the process involved in electroplating a silver coin.arrow_forwardUsing standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG° for the following redox reaction. Round your answer to 3 significant digits. 6CI (aq)+2Cr0 (aq)+8H,0 (1) → 3Cl, (g)+2Cr(OH), (s) + 10OH (aq)arrow_forward
- Mn(s)|Mn2+(aq)||Cr3+(aq)| Cr(s) Calculate the cell potential at 25oC if the concentration of Mn2+(aq) is 2.40 M and the concentration of Cr3+(aq) is 0.500 M. Report your answer to 3 significant figures. Use theStandard Reduction Potentials Table as needed.arrow_forwardCalculate the equilibrium constant, K, for the reaction shown at 25 °C. Fe+(aq)+B(s) + 6 H, O(1) → Fe(s) + H,BO,(s) + 3 H,O*(aq) The balanced reduction half-reactions for the equation and their respective standard reduction potential values (E") are Fe3+ (aq) + 3 e- → Fe(s) H,BO, (s) + 3 H,0*(aq) + 3 e- – B(s) + 6 H, O(1) E' = -0.04 V E' = -0.8698 V K =arrow_forwardI need hand written solution only otherwise I will down votearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY