
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:3. Estimate Eº for the half-reaction
2H₂O + 2e → H₂ + 2OH-
given the following values of AG :
H₂O(l) = -237 kJ/mol
H₂(g) = 0.0
OH(aq) = -157 kJ/mol
e = 0.0
Compare this value of E° with the value of E° given in Table 18.1
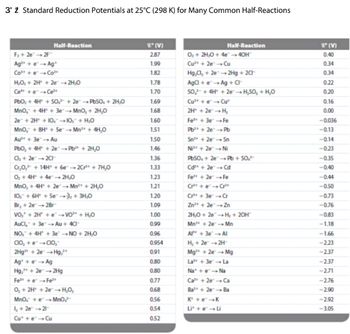
Transcribed Image Text:3' Ž Standard Reduction Potentials at 25°C (298 K) for Many Common Half-Reactions
Half-Reaction
F₂+2e-2F
Ag+ e → Ag
coi te co
H₂O₂ + 2H+ 2e → 2H₂O
Ce +e→Ce"
PbO₂ + 4H+SO+2e →PbSO. + 2H₂O
MnO, + 4H+ + 3e →MnO₂ + 2H₂O
2e + 2H+10 →10 + H₂O
MnO, +8H + 5e →Mn² + 4H₂O
Au + 3e → Au
PbO₂ + 4H+ 2e →Pb² + 2H₂O
Cl₂ +2e2C1-
Cr₂0,² + 14H+ + 6e →2Cr³+ + 7H₂0
O₂ + 4H+ 4e → 2H₂O
MnO₂ + 4H+ + 2e →Mn²+ + 2H₂O
10₂ + 6H + 5e →→₂ + 3H₂O
Br₂ +2e2Br
VO₂ + 2H+ e→VO² + H₂O
AuCl₂ + 3e-Au + 4C
NO₂ + 4H+ 3e→NO + 2H₂O
CIO₂ + e→CIO,
2Hg2+ 2e → Hg₂²⁰
Ag+ e → Ag
Hg₂+ 2e →→ 2Hg
Fe +eFe²+
O₂ + 2H+ 2e → H₂O₂
MnO, + e→→MnO²-
1₂ + 2e → 21-
Cut + e-Cu
8° (V)
2.87
1.99
1.82
1.78
1.70
1.69
1.68
1.60
1.51
1.50
1.46
1.36
1.33
1.23
1.21
1.20
1.09
1.00
0.99
0.96
0.954
0.91
0.80
0.80
0.77
0.68
0.56
0.54
0.52
Half-Reaction
O₂ + 2H₂O + 4e →40H™
Cu²+ 2e → Cu
Hg,Cl₂ + 2e → 2Hg + 2C1-
AgCl+eAg + Cr
SO2 + 4H+ 2e → H₂SO₂ + H₂O
Cu²+eCut
2H + 2e → H₂
Fe + 3e-Fe
Pb²+ + 2e →→ Pb
Sn² +2e → Sn
Ni²+ + 2e-Ni
PbSO4 + 2e →→ Pb + 50,²
Cd²+2e →→ Cd
Fe + 2e → Fe
Cr³+ + e→Cr³
Cr³+ + 3e-Cr
Zn²+2e → Zn
2H₂O + 2e →→H₂ + 20H™
Mn +2e →→Mn
AP+3e Al
H₂ + 2e → 2H™
Mg2+2e → Mg
La" + 3e-La
Na + e-Na
Ca²+2e →→ Ca
Ba²+2e-Ba
K' + e-K
Li' + e-Li
gº (V)
0.40
0.34
0.34
0.22
0.20
0.16
0.00
-0.036
<-0.13
<-0.14
-0.23
<<-0.35
-0.40
-0.44
-0.50
-0.73
-0.76
-0.83
-1.18
-1.66
-2.23
-2.37
-2.37
<-2.71
-2.76
-2.90
-2.92
-3.05
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the following half reaction: Na (ag) + e → Na(s). For this reaction, E°(red) = -2.7 V. If this reaction is tripled so that 3 Na ions are reduced to 3 Na atoms, what is the new E°(red)? ->arrow_forwardConsider the galvanic cell based on the following half-reactions. Ag+ + e− → Ag ℰ° = +0.80 V Fe2+ + 2 e− → Fe ℰ° = −0.44 V (a) Determine the overall cell reaction and calculate ℰ ° cell . (b) Calculate ΔG° and K for the cell reaction at 25°C. (c) Calculate ℰcell at 25°C when [Ag+ ] = 1.0 ✕ 10−4 M and [Fe2+ ] = 1.0 ✕ 10−2 M.arrow_forwardCalculate the K and AG for the following reaction at 25 °C: Mg(s) + Pb2+(aq)=Mg2+(aq) + Pb (s) Note: Reference the Standard reduction potentials at 25 °C and Fundamental constants tables for additional information. Part 1 of 2 Round your answer to 3 significant digits. Кс Part 2 of 2 G Round your answer to 4 significant digits. AG° kJ molarrow_forward
- What is E°cell for the following reaction? Is this reaction spontaneous under standard conditions? Cu2+(aq) + Sn2+(aq) → Cu(s) + Sn4+(aq) Cu2+(aq) + 2e– → Cu(s) E° = 0.34 V Sn4+(aq) + 2e– → Sn2+(aq) E° = 0.13 V Group of answer choices –0.21 V, nonspontaneous 0.21 V, spontaneous 0.21 V, nonspontaneous –0.21 V, spontaneousarrow_forwardConsider the following half-reactions. Cl2(g) + 2 e– → 2 Cl–(aq) E° = +1.36 V Ag+(aq) + e– → Ag(s) E° = +0.80 V Cu2+(aq) + 2 e– → Cu(s) E° = +0.34 V Sn2+(aq) + 2 e– → Sn(s) E° = –0.14 V Al3+(aq) + 3 e– → Al(s) E° = –1.66 V Which of the following species will reduce Cu2+(aq) ion? Ag(s) and Sn^2+(aq) Cl^-(aq) and Ag(s) Cl2(g) and Ag^+(aq) Sn(s) and Al(s) Sn^2+(aq) and Al^3+(aq)arrow_forwardDetermine E for the half-reaction.arrow_forward
- The standard state cell potential for the reaction below is 1.030 V. Zn(s) + Hg,Cl,(s)→ ZnCl,(aq) + 2Hg(1) Calculate the standard state Gibb's energy change (in kJ) at 298K for the reaction. O -99.4 kJ O -199 kJ O 199kJ O 99.4 kJarrow_forwardGiven the following two half reactions, Cd2+(aq) + 2e- → Cd(s) E˚ = -0.40 V 2 Ag+(aq) + 2e- → 2 Ag(s) E˚ = 0.80 V determine E˚ and the spontaneity of the following reaction: 2 Ag+(aq) + Cd(s) → 2 Ag(s) + Cd2+(aq)arrow_forwardFind K for the following reaction at at 25°C. E cell = -0.10 V Cd²+ (aq) + Fe(s) Cd(s) + Fe2+ (aq) 4.2 x 10-4 O 1.2 x 10-5 1.2 x 105 4.2 x 1010arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY