
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Use Newman’s projection to predict the major product for an E2 reaction of the substrate shown below, and also draw out how the final product will look li
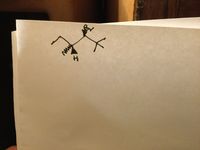
Transcribed Image Text:The image displays a molecular structure, which is characteristic of organic chemistry. Here is a detailed breakdown of the structure:
- **Central Carbon Atom (Chiral Center):** The central point of the diagram represents a carbon atom, which acts as a chiral center.
- **Bromine (Br) Group:** Attached to the central carbon is a substituent labeled "Br," indicating a bromine atom.
- **Hydrogen (H) Atom:** Another substituent attached to the central carbon is labeled "H," representing a hydrogen atom.
- **Wedge and Dash Notation:**
- The **solid wedge** bond indicates that the bromine is projecting outwards, towards the observer.
- The **dashed line** bond, often referred to as a "dash," signifies that the hydrogen is projecting away from the observer, indicating stereochemistry.
- **Other Lines:** The remaining two lines represent additional substituents or extensions in the molecular structure, but without specific labels, they represent unspecified groups or carbon chains.
This diagram is a typical representation used to depict the three-dimensional spatial arrangement of atoms around a stereocenter in molecules, which is crucial for understanding chiral molecules in organic chemistry.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 9. Draw the mechanism for the following SN1 reaction x Br EtOHarrow_forwardDraw Zaitsev and Hofmann products that are expected when each of the following compounds is treated with a strong base to give an E2 ELIMINATION product. Hint: Some compounds cannot produce both elimination products because they do not have two beta-hydrogen atoms: in these cases only ONE product will be possible. In cases when neither elimination products are possible, there will be NO REACTION. ? O A O O O B U Br t Zaitsev Hofmann only ONE product is possible B only ONE product is possible Hofmann Zaitsevarrow_forwardType of substrates undergo SN2 & SN1 reactions & 3 examples SN2 SN1arrow_forward
- Determine which mechanism below is the most correct. Be sure to look at whether the most acidic H is reacting, if the direction of the arrows are correct, and if the correct products are formed based on the mechanism. If the correct answer is Option B, then explain why the other options are incorrect? Explain each option that is incorrect individually.arrow_forwardDraw the product of the E2 reaction shown below. Include the correct stereochemistry. Ignore any inorganic byproducts. CH3 H. Ph H CH2CH3 Br NaNH2 Barrow_forwardI need help drawing the product of this reaction. The starting material is vanillin and it is being treated with SO3 & H2SO4 in order to generate a product with the formula C8H8SO6.arrow_forward
- Draw the product of the E2 reaction shown below. Include the correct stereochemistry. Ignore any inorganic byproducts. H. H3CH₂C CH3 Br NaNHz LOCH3 H Qarrow_forwardNitesharrow_forward5. Draw the mechanism of the following SN1 reaction and the products with Stereochemistry. Draw the intermediate. Br ||||| H :O: Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY