
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
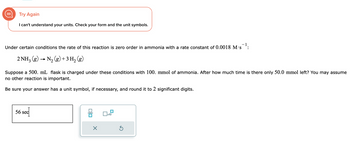
Transcribed Image Text:Try Again
I can't understand your units. Check your form and the unit symbols.
Under certain conditions the rate of this reaction is zero order in ammonia with a rate constant of 0.0018 M.S
2 NH3(g) → N₂(g) + 3 H₂ (g)
Suppose a 500. mL flask is charged under these conditions with 100. mmol of ammonia. After how much time is there only 50.0 mmol left? You may assume
no other reaction is important.
Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.
56 sec
x10
Ś
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Under certain conditions the rate of this reaction is zero order in dinitrogen monoxide with a rate constant of 0.0022 Ms: 2N,0 (g) - 2N, (g) +0, (g) Suppose a 400. mL flask is charged under these conditions with 500. mmol of dinitrogen monoxide. After how much time is there only 250. mmol left? You may assume no other reaction is important. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits. 10 Ararrow_forwardAt a certain temperature the rate of this reaction is first order in HI with a rate constant of 1.13 s : 2HI (g) → H2 (g) +I, (g) Suppose a vessel contains HI at a concentration of 1.01 M. Calculate the concentration of HI in the vessel 0.730 seconds later. You may assume no other reaction is important. Round your answer to 2 significant digits. M Ox10arrow_forwardUnder certain conditions the rate of this reaction is zero order in hydrogen iodide with a rate constant of 0.0041 M's : 2 HI(g) → H2(g)+I,(g) Suppose a 4.0 L flask is charged under these conditions with 300. mmol of hydrogen iodide. After how much time is there only 150. mmol left? You may assume no other reaction is important. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits. x10arrow_forward
- - 1 Under certain conditions the rate of this reaction is zero order in hydrogen iodide with a rate constant of 0.0020 M·s 2 HI (g) → H, (g) + I, (g) Suppose a 4.0 L flask is charged under these conditions with 150. mmol of hydrogen iodide. How much is left 5.0 s later? You may assume no other reaction is important. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.arrow_forwardUnder certain conditions the rate of this reaction is zero order in hydrogen iodide with a rate constant of 0.0044 M's : 2 HI (g) - H, (g) +I, (g) Suppose a 3.0 L flask is charged under these conditions with 400. mmol of hydrogen iodide. How much is left 8.0 s later? You may assume no other reaction is important. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits. x10arrow_forwardUnder certain conditions the rate of this reaction is zero order in hydrogen iodide with a rate constant of 0.0092 M-s : 2 HI (g) - H, (g) +L, (g) Suppose a 450. mL flask is charged under these conditions with 150. mmol of hydrogen iodide. After how much time is there only 75.0 mmol left? You may assume no other reaction is important. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits. dlo x10 8. 미arrow_forward
- A chemistry graduate student is studying the rate of this reaction: 2C1₂05 (g) → 2Cl₂ (g) +50₂ (g) She fills a reaction vessel with C1₂05 and measures its concentration as the reaction proceeds: time [C1,05] (seconds) 0 0.400M 10. 0.263 M 20. 0.173 M 30. 0.114M 40. 0.0751M Use this data to answer the following questions. Write the rate law for this reaction. x10 Calculate the value of the rate constant k. X Round your answer to 2 significant digits. Also be sure your answer has the correct unit symbol. rate = k k = 0 Ś ?arrow_forwardThe reaction of NOBr(g) to form NO(g) and Br2(g) is second order: 2NOBr(g) → 2NO(g) + Br₂(g) The rate constant is 0.556 L mol-¹ s¹ at some temperature. If the initial concentration of NOBr in the container is 0.21 M, how long will it take for the concentration to decrease to 0.028 M? t = Sarrow_forwardConsider this reaction: 2HI (g) → H, (g) +I, (g) At a certain temperature it obeys this rate law. rate =(0,465 s)[HI Suppose a vessel contains HI at a concentration of 0.540M. Calculate the concentration of HI in the vessel 2.80 seconds later. You may assume no other reaction is important. Round your answer to 2 significant digits.arrow_forward
- 2 3 4 =5 #1 6 7 Under certain conditions the rate of this reaction is zero order in hydrogen iodide with a rate constant of 0.0040 M-s¹: 2 HI (g) → H₂(g) + 1₂ (g) Suppose a 5.0 L flask is charged under these conditions with 300. mmol of hydrogen iodide. How much is left 4.0 s later? You may assume no other reaction is important. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits. S A S ? Continue Submit 00 Xarrow_forwardConsider this reaction: 2H1 (g) → H₂(g) +1₂(e) At a certain temperature it obeys this rate law. rate = (0.345-¹) [HI] Suppose a vessel contains HI at a concentration of 0.730M. Calculate how long it takes for the concentration of HI to decrease by 86.0%. You may assume no other reaction is important. Round your answer to 2 significant digits. 0. 08arrow_forwardUnder certain conditions the rate of this reaction is zero order in dinitrogen monoxide with a rate constant of 0.0086 M's : 2 N,0 (g) → 2 N2 (g) + 0, (g) Suppose a 300. mL flask is charged under these conditions with 100. mmol of dinitrogen monoxide. How much is left 10. s later? You may assume no other reaction is important. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits. x10 미arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY