
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:- 1
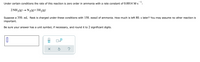
Under certain conditions the rate of this reaction is zero order in ammonia with a rate constant of 0.0014 M•s
2 NH3(g) → N2(8)+3H2(g)
Suppose a 350. mL flask is charged under these conditions with 150. mmol of ammonia. How much is left 80. s later? You may assume no other reaction is
important.
Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.
x10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The balanced chemical equation for the reaction between iodine vapour and fluorine gas is: 3I2(g) + 6F2(g) → 2IF5(g) + I 4F 2(g) A chemist measures the rate at which iodine vapour is consumed to be 4.5 × 10–4 mol/(L•s). At what rate is F2(g) consumed?arrow_forwardIn a certain chemical reaction, substance A combines with substance B to form substance Y. At the start of the reaction, the quantity of A present is a grams, and the quantity of B present is b grams. Assume a < b and y ≤ a. At time t seconds after the start of the reaction, the quantity of Y present is y grams. For certain types of reactions, the rate of the reaction, in grams/sec, is given by Rate = k(a - y)(b − y), - where k is a positive constant. 1. Sketch a graph of the rate against y. For what values of y is the rate nonnegative? Give your answer as a union of intervals, e.g., (- infinity,-a] U (a, 2b) YE 2. Use your graph to find the value of y at which the rate of the reaction is fastest. У =arrow_forwardConsider the reaction: N₂(g) + 3H₂(g) → 2NH, (g) Suppose that a particular moment during the reaction, molecular hydrogen is reacting at a rate of -0.0540 formed? Be sure your answer has the correct number of significant digits. 0x100M S 10 X M At what rate is ammonia beingarrow_forward
- Chlorine dioxide, CIO₂, is a reddish-yellow gas that is soluble in water. In basic solution it gives CIO3 and ClO₂ ions. 2 CIO₂(aq) + 2 OH(aq) → CIO3(aq) + ClO₂ (aq) + H₂O(1) To obtain the rate law for this reaction, the following experiments were run and, for each, the initial rate of reaction of ClO₂ was determined. Use the data here to determine the order of the reaction for CIO₂ and OH, and the value of the rate constant. [CIO2]init [OH Jinit Initial Rate, mol/(Los) Exp. 1 0.030 Exp. 2 0.060 Exp. 3 0.030 Order for CIO₂: Order for OH": Rate constant = 0.030 0.030 0.060 0.0062 0.0248 0.0124arrow_forwardConsider the reaction: N₂(g) + 3H₂(g) M Suppose that a particular moment during the reaction, molecular hydrogen is reacting at a rate of -0.0820. At what rate is ammonia being formed? Round your answer to 3 significant digits. I M 2NH₂ (g) S x10 X Ś Sarrow_forwardUnder certain conditions the rate of this reaction is zero order in dinitrogen monoxide with a rate constant of 0.0051 M.s 1: 2N₂O(g) →2N2(g) +0₂ (g) Suppose a 5.0 L flask is charged under these conditions with 250. mmol of dinitrogen monoxide. After how much time is there only 125. mmol left? You may assume no other reaction is important. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits. = X x10arrow_forward
- Under certain conditions the rate of this reaction is zero order in hydrogen iodide with a rate constant of ·0.0040Ms−1:2HI(g)→H2(g)+I2(g) Suppose a 300.mL flask is charged under these conditions with 500.mmol of hydrogen iodide. After how much time is there only 250.mmol left? You may assume no other reaction is important. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.arrow_forwardUnder certain conditions the rate of this reaction is zero order in ammonia with a rate constant of 0.0029 M·s : 2 NH3 (g) → N, (g)+ 3 H, (g) Suppose a 3.0 L flask is charged under these conditions with 400. mmol of ammonia. After how much time is there only 200. mmol left? You may assume no other reaction is important. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits. х10arrow_forwardUnder certain conditions the rate of this reaction is zero order in ammonia with a rate constant of 0.0032 M's : 2 NH, (g) → N2 (g) +3 H, (g) Suppose a 3.0 L flask is charged under these conditions with 400. mmol of ammonia. How much is left 10. s later? You may assume no other reaction is important. olo Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits. Ararrow_forward
- Consider the reaction: 2N205(g)4NO2(g) + O2(g) At a particular temperature, the rate constant is 3.81 x 10s. If the initial concentration of N205 is 0.0212 M, calculate the concentration (in M) of N,05 after 882 seconds at this particular temperature. Please express the answer in scientific notation. If the answer is 2.5 x 102, express the answer as 2.5E-2. O Respond with the correct number of significant figures in scientific notation (Use E notation and only 1 digit before decimal e.g. 2.5E5 for 2.5 x 10") Type your numeric answer and submitarrow_forwardPlease don't provide handwritten solution...arrow_forwardA reaction proceeds according to the following equation: A + 3B → 2C + 2D At an early stage of the reaction, conc. A = 1.191 M and 6.06 minutes later, conc. A= 0.949 M. What is the average rate of the reaction during this time period, expressed in units of M·s-1?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY