
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
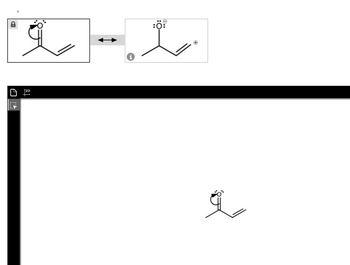
Transcribed Image Text:**Educational Content: Resonance Structures of Acetone Enolate**
In organic chemistry, understanding the concept of resonance structures is essential for grasping how electrons are distributed in molecules. This example illustrates the resonance structures of the acetone enolate ion.
### Resonance Structures of Acetone Enolate
#### Top Diagram:
**Left Structure:**
This structure depicts an enolate form of acetone. It shows a carbon-carbon double bond adjacent to a negatively charged oxygen atom with lone pairs of electrons. The negative charge indicates an excess electron density on the oxygen atom.
**Right Structure:**
This resonance form shows the distribution of the negative charge. Here, the double bond between the carbons has shifted, resulting in a single bond between the carbons and a double bond between the central carbon and oxygen. The oxygen atom now carries a negative formal charge, while the adjacent carbon holds a positive formal charge.
These two structures are connected by a double-headed resonance arrow, indicating that the true nature of the acetone enolate ion is a hybrid of these two contributors.
### Description of the Bottom Image
**Diagram:**
The bottom image replicates the enolate form of acetone, illustrating one of the resonance structures identical to the left structure in the top diagram. It effectively shows the negative charge on the oxygen, the carbon-carbon double bond adjacent to it, and the position of the hydrogen atoms.
#### Explanation of Resonance:
These structures demonstrate the resonance delocalization of the negative charge. In the actual molecule, the electron density is not confined to one position but is instead shared across the structure, making the molecule more stable than any individual resonance form.
This concept is crucial for understanding the reactivity and stability of enolates and other resonance-stabilized intermediates in organic reactions.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ask Laftan Anlamaz - Episode O St. John's University - My Appl X A ALEKS - Iffat Khan - Learn O Mỹ Applicatio -> A www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lijkPWvZOZLqKt1FLIq7wcPWKzBYGfE9IMFjjocijBjuQK3I O ATOMS, IONS AND MOLECULES Naming ionic compounds with common polyatomic ions Fill in the name and empirical formula of each ionic compound that could be formed from the ions in this table: Some ionic compounds cation anion empirical formula name of compound K Clo, 4+ Pb NO, so 2- NH,arrow_forwardThe following picture describes a/an relationship. 10 6. 7. 4 2. 200 300 400 500 600 700 800 900 1000 100arrow_forwardto Johnston Commun X Rh Blackboard Collaborate Ultra - x > General Psychology-Fall 2021 A ALEKS - Griffin Barden - Learn + A www-awa.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IJczzdcvSCzsqTCIDqNGV3bKqhMfPmUcQ4ENkmiXn9QCwgeDPDkQ06yszYWESPcekwLO-Qg619rekU7404HgFAGbEZa Dr080?1 oBw7QYjlbavbSPXtx-YCjsh_7mMmrq#item O THERMOCHEMISTRY Griffin Calculating a molar heat of reaction from formation enthalpies Using the table of standard formation enthalpies that you'll find under the ALEKS Data tab, calculate the reaction enthalpy of this reaction under standard conditions: 2C,H;(g)+70,(9)→4 CO,(g)+6H,O() Round your answer to the nearest kJ.arrow_forward
- A ALEKS-Lave Atthawadi-Le www-awu.aleks.com/alekscgi/x/isl.exe/1o_u-IgNsikr7j8P3jH-IvTqeviKFP6W0cqJcWJdIACROQwyw24GWHInZ2njVjs1cHQJvinqDviXxn9bAVrFaae-... Reading Schedule 24 Solubility and 18.3 Gibbs Free E.5.3 Enthalpies of... 18.5 Gibbs Free E... Reading Schedule O ACIDS AND BASES Calculating the composition of a buffer of a given pH E esc Slide 46 of 105 A chemistry graduate student is given 100. mL of a 0.30M acetic acid (HCH,CO₂) solution. Acetic acid is a weak acid with K-1.8 x 105. What mass of KCH,CO, should the student dissolve in the HCH,CO₂ solution to turn it into a buffer with pH = 4.62? 0 You may assume that the volume of the solution doesn't change when the KCH, CO₂ is dissolved in it. Be sure your answer has a unit symbol, and round it to 2 significant digits. Explanation 7 A Notes Z @ 2 Check 0.9 Comments S # 3 H X :: E D 3 4 C 1110+ > R F 5 V I T G 79% 6 3 B MacBook Pro Y 4 19.6 Reduction Po... Y SOLUTION: The le... Math 115 W-S Fall... Po... 19.6 Reduction I'm H…arrow_forwardALEKS - Chandni Singh My Questions | bartleby X 9 CHEM 1A General Chemistry 726 X NetTutor Tutoring https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IBd6bRWrqRgBHIfQgBpPcO88HtLzLSjbnicWjKNFwVA6GBjSaytD.. O CHEMICAL REACTIONS Chandni Percent yield of chemical reactions Aqueous hydrobromic acid (HBr) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium bromide (NaBr) and liquid water (H,O). If 0.0311 g of water is produced from the reaction of 0.81 g of hydrobromic acid and 0.23 g of sodium hydroxide, calculate the percent yield of water. Be sure your answer has the correct number of significant digits in it. 圖 % olo Explanation Check © 2021 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility 12:06 AM 2 Type here to search 60°F Clear ^ Q») ENG 9/16/2021arrow_forwardALEKS - Rafia Riaz - Knowledge CX M Gmail T LI www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lvdw7xgLCkqMfg8yaFKbD9GafJstkYLIJnusU-prelf7ZFNxcVAY-BM1i3u9X-haj|PV32e8Emd_XSqOPnmRsQgU5WQJ-r7s?1oBw7Q... Q College information G cradles by sub urba... a [Beauty of Joseon]... Labcorp | PreCheck YouTube Maps + Type here to search GTranslate = Module Knowledge Check K The pH of a 0.14M solution of pentanoic acid (HC3H₂O₂) is measured to be 2.85. Calculate the acid dissociation constant K of pentanoic acid. Be sure your answer has the correct number of significant digits. 0 News I Don't Know E Submit Question 1 X garlands - Google S... rasta tracksuit - Go... Rafia V olo Är © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility (?? 53°F Mostly cloudy J x 5:36 PM 4/5/2023 : Other bookmarks Marrow_forward
- Moore 5e_C OWLV2 | Onli X Q ☆ * CE w.com/ilrn/takeAssignment/takeCovalentActivity... [Review Topics] [References] isms ots 2req Use the References to access important values if needed for this question. In a study of the conversion of methyl isonitrile to acetonitrile in the gas phase at 250 °C Visited 1 pts 2req CH3NC(g)- >CH3CN(g) 1 pts 2req the concentration of CH NC was followed as a function of time. It was found that a graph of In[CH3NC] versus time in seconds gave a 1 pts 2req straight line with a slope of -3.96x10-3 s and a y-intercept 52. 1 pts 2req Based on this plot, the reaction is v order in CH,NC and the rate constant for the reaction is 1 pts 2reg 1 pts 2reg 1 pts 2reg Submit Answer Retry Entire Group 9 more group attempts remaining Previous Next Email Instructor Save and Exit Cengage Learning | Cengage Technical Support 52% 1:41 PM 1/22/2021arrow_forward- (17 · (17 Fra Fra ge edu/courses/159861/files?preview=69941104 الولايات المتحدة القن... 0 Ns G Gmail als Science Problem Set 2.pdf W F2 # 2 ۲ 3 r b Ac E 80 F3 D 16 $ Why You Don't Ne... 4 E (PI ASU Fil W Juj (PL R Page of 2 | 0 | 9 al K Û F9 ) O PSU X 0 [ O A Alternative خ F10 . La P * Varrow_forwardSt. John's University - My Ap X A ALEKS - Iffat Khan - Knowled x 9 Award Package By Aid Year A Apply fe i www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lijkPWvZoZLqKt1FLIq7wcPWKzBYGfE9IMFjOOhkzHKG7r9iWmkWtp_VVI7DJegbC Knowledge Check Question 17 An unknown compound has the following chemical formula: N,O3 where x stands for a whole number. Measurements also show that a certain sample of the unknown compound contains 2.5 mol of nitrogen and 3.69 mol of oxygen. Write the complete chemical formula for the unknown compound. I Don't Know Submitarrow_forward
- -> https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQUHIQg6bJxmeSyVpHOEB1plef9xyC5Ca9QI15ULF571w... A to tho subject) - maryhan X M <3- mary.hamilton@s X To 3 1 O ADVANCED GENERAL CHEMISTRY Calculating an equilibrium constant from an equilibrium.. 3/5 Hydrogen and chlorine react to form hydrogen chloride, like this: H,(g) + Cl,(g) → 2 HCl(g) Also, a chemist finds that at a certain temperature the equilibrium mixture of hydrogen, chlorine, and hydrogen chloride has the following composition: compound pressure at equilibrium H2 62.7 atm Cl 35.7 atm HCl 85.0 atm Calculate the value of the equilibrium constant K, for this reaction. Round your answer to 2 significant digits. d. K D x10 Eſplanation Check O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Usel Privacy Center | Acce P W F7 F8 F9 F10 F11 F12 PriScr risert Delete FA FS F6arrow_forwardA ALEKS-Lara Althawadi - Lear X G 3.0 sig figs - Google Search X G 0.011 sig figs - Google Search x + www-awu.aleks.com/alekscgi/x/lsl.exe/10_u-IgNsikr7j8P3JH-IvTqeviKFP6W0cqJcWJdIACROQwyw24GWHInL27j0m-BbhK772LRC-WPOIJB064JOgeD... ✰ 18.3 Gibbs Free E... 5.3 Enthalpies of... 18.5 Gibbs Free E.... Reading Schedule 19.6 Reduction Po... Y SOLUTION: The le Math 115 W-S Fall.... C Solubility and.... SC O ACIDS AND BASES Calculating the pH at equivalence of a titration A chemist titrates 160.0 mL of a 0.5839M benzoic acid (HC,H,CO₂) solution with 0.4219M KOH solution at 25 °C. Calculate the pH at equivalence. The p K of benzoic acid is 4.20. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of KOH solution added. pH = 0 Explanation Check Methods for Measuring the pH of an Aqueous Slide 28 of 104 Notes X Click to add notes 19 Comments S P DA :: R 5 + 63% 53 T 6 G 00000 3/5 I'm B h. Get…arrow_forwarddmentn Leaming Inyinor X 6Willen her n Ine aJLAO ohn the eProperties and Changes of MalteM * 12.app.edmentum.com/assessments delivery/ua/mt/launch/495124 38/45424663/al 0cM6lyA Au/Wie Next O Properties and Changes of Matter: Mastery Test 1. Select the correct answer. Which type of property can be observed only by changing a substance into a different substance? intensive property OB. extensive property OC. physical property OD. chemical property Reset Next 2022 Edmentum. All rights reserved. Type here to searcharrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY