
Fundamentals Of Analytical Chemistry
9th Edition
ISBN: 9781285640686
Author: Skoog
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:molar mass of na2s2o3-Google X
October 1, John Searle "Minc @ x +
www-awu.akeks.com/alekscgi/x/Isl.exe/1o u-lgNslkr7j8P3jH-lix5uFZIVj2iEJjąd1WoxT77ErtzZpGbzESW6u_S5FZDV02WDPG OKqpiLH65e00TK3cK7TrLikOsdoZA3aRgGhL..
O ADVANCED MATERIAL
三
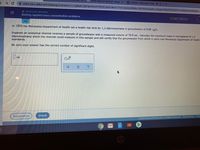
Solving applied mass concentration problems
In 1993 the Minnesota Department of Health set a health risk limit for 1,2-dibromoethane in groundwater of 4.00 ng/L.
Suppose an analytical chemist recelves a sample of groundwater with a measured volume of 78.0 mL. Calculate the maximum mass in micrograms of 1,2-
dibromoethane which the chemist could measure in this sample and still certify that the groundwater from which it came met Minnesota Department of Health
standards.
Be sure your answer has the correct number of significant digits.
ロ
x10
Explanation
Check
2020 McGraw-Hill Education. All Rights Reserved. Terms of Use | Prie
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- AClasswork for Previti-Bth Gre de X EHalagh Reed-Rocket Sledder Se x Submit Conservation of Mass M X G alun app.teachemmade.com/fill/e245a834-aled-47e1-9791-924dee991f03 K! OCQQ T TI 14 Ag +S-AG2S Conservation of Mass Draw enough molecules to conserve mass in each reaction aluminum bromine -> aluminum bromide Br Al Al Br Al Al Br Br Br Draw more bromine How many of cach atom are in the rtactants? How many of cach atom are in the products? A aluminum Br bromine A auminum bromine Balanced cquation sodium water sodium hydroxide hydrogenarrow_forwardMaps G Google c Compounds i Which has the highest boiling point? Multiple Choice .docx xternal_browser=0&launchUrl=https Grammarly My Citation Lists | E... C Solved: Chapter 12... diesel fuel (composed mostly of C15H32-C18H38) gasoline (composed mostly of C5H12-C12H26) kerosene (composed mostly of C12H26-C16H34) All of these are petroelum products, so all have the same boiling point. < Prev 18 of 4 O O Darrow_forwardPT McGraw Hill Campus Cn [11 O ATOMS, IONS AND MOLECULES Finding atomic mass from isotope mass and natural abundance isotope 74°F 100. Calculate the Molar Mass of Acet X+ https://www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-liG1Sp4C1qHE9vg-2-exSXq493r_tJ2gZs0a-_xkRSR8a7T-... A robot spacecraft returned samples from the planetesimal 98765 ALEKS, located in the outer Solar System. Mass-spectroscopic analysis produced the following data on the isotopes of molybdenum in these samples: Mo 99.91 Mo 95.90 x A ALEKS - Elizabeth Galaz - Learn x mass relative (amu) abundance Mo 0 96.2% Explanation 3.8% Use these measurements to complete the entry for molybdenum in the Periodic Table that would be used on 98765 ALEKS. Round your entry for the atomic mass to 3 significant digits. Caution: your correct answer will have the same format but not necessarily the same numbers as the entry for molybdenum in the Periodic Table we use here on Earth. X Check A A AB Search 3/5 hp A @ 0 LE Elizab…arrow_forward
- Lüiza Pai >X U Meet - sX 22 Escola A x LA Meet- FX LA Meet- X Particip X google.com/presentation/d/1eWnH2zEnF-fMFWWp4Clvpch3cmrebrO5QTYSU96nNww/edit#slide=id.g3da23e319d_0_. MY 3) - G9 The Atom at Slide Arrange Tools Add-ons Help Last edit was 20 minutes ago D Present 回▼ O 田 Background Layout - Theme Transition . 1 2 3 4 6. | 7 8 | 9 Label Carbon's Periodic Table Symbol ANSWER BANK 6. Mass Number C Average Atomic Mass Atomic 12.011 Number Leave one here! ODRAG ck to add speaker notes PORarrow_forwardexe/lo_u-IgNslkr7j8P3ji Qs_dp5pR4ENzvdYC-70kXyMz36BqJhw3sVP80GQVcR8H8EYh1OU3pQMbCr ourse Home Login | Student Veri... Logout 5 MyProgrammingLab Imported From IE O ITE MEASUREMENT AND MATYER Predicting and naming ionic compounds formed by two elements Decide whether each pair of elements in the table below will form an ionic compound. If t formed in the spaces provided. empirical formula of ionic compound element #1 element #2 Forms ionic compound? name of ionic compound lithium lodine yes O no calcium sulfur Oyes no fluorine sulfur yes cesium uniseube O yes noarrow_forwardCO 5 hway Hydrate Exp x lab 10 empirical formu X C Lab 11 - Atomic Finger X G periodic table-Google X C ALEKS ALEKS- Courtney Wh X New Tab i www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-lijulplKKWv4bwenmyrLYoBHBAAsJtmzj53GErnx_viTdHbIGMF9jvgkSu_nrizREmdQXHPJbMzq0mOrXJcuonZITQhB66q?1o8w7 O CHEMICAL REACTIONS C 315 Theoretical yield of chemical reactions Gaseous methane (CH) reacts with gaseous oxygen gas (0,) to produce gaseous carbon dioxide (CO,) and gaseous water (H,0). What is the theoretical yield of water formed from the reaction of 12.7 g of methane and 34.9 g of oxygen gas? Be sure your answer has the correct number of significant digits in it. Explanation Check ©2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center | Accessibility II0 -> 24 4. %23 7. 8. 3. n 4 ctrl altarrow_forward
- D2L Homepage - S X (26) Pinterest x y bartleby - Yahox b Success Confi x с app.101edu.co < + M Your Chemistry x b Answered: Que x Time's Up! Determine the mass in grams of 3.30 × 1021 atoms of arsenic. (The mass of one mole of arsenic is 74.92 g.) Aktiv Chemistr X b Success Confi x 1 4 7 +/- 5 New Tab 2 3 8 g 6 9 G 0 x + Submit X C x 100arrow_forwardally. * Question Completion Status: QUESTION 1 During the experiment, the mass of original sample of compound was 2.965 g, and the mass of copper obtained was 1.006 g. Based on these numbers, calculate the mass of chlorine in the original sample in grams. Include the unit and three decimal places in your answer. QUESTION 2 6. If 1.045 g of copper was obtained in the experiment, how many moles (mol) of copper is this? Include the unit and five decimal places in your answer. QUESTION 3 During the experiment, a student calculated the mass of chlorine in the compound to be 1.186 g. How many moles (mol) of chlorine are in the Save All Answers Click Save and Submit to save and submit. Click Save All Answers to save all answers. DELL IIarrow_forwardpshotid=2199... NDTAP Q Search this cou Use the References to access important values if needed for this question. Calculate the number of atoms of hydrogen (H) in 394 cm³ of the colorless gas butane at 25 °C and atmospheric pressure, where its density is 2.38×10³ g cm-3. The molecular formula of butane is C4H10- H atoms Submit Answer 4 question attempts remainingarrow_forward
- mage tesuit for sur ris-Molar Mass Worksheet TURE ew Insert Format Tools Add-ons Help Last edit + BIUA 75% Normal text Arial 14 Find the molar mass of the following chemicals: Sodium (Na) Calcium (Ca) Xenon (Xe) Gadolinium (Gd) Uranium (U) O'H H,SO NaOH CON) CaH18arrow_forwardPayalarrow_forward← → CO www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3jH-JgXZp57itWHhRgilOD 0 Email M Gmail YouTube Maps MyCSU-Columbus... B Homepage - Georg... W Microsc O STATES OF MATTER Calculating mass percent composition V A chemist mixes 74.9 g of water with 80.1 g of 2-methylpyrazine and 34.8 g of acetic acid. Calculate the percent by mass of each component of this solution. Be sure each of your answer entries has the correct number of significant digits. component mass percent x10 water 0 % S ? 2-methylpyrazine 0% acetic acid 0% ||| esc Explanation Check Type here to search f2 ? f3 f4 X O i f5 16 a & O dip 17arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
