
Fundamentals Of Analytical Chemistry
9th Edition
ISBN: 9781285640686
Author: Skoog
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Question
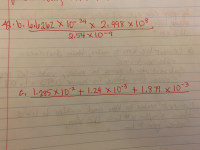
Transcribed Image Text:4 W
A.b.l0l0a2X 103 2.998 x10
25410-9
VI
of pnat r roona9
cow of of s/omad
ot
.Smy
Zo sotary
EP
C A85I0
SUI
1.24 x 10+ 1,871 x10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- ALEKS - Jacqueline Hoppenrey x G convert mg to g - Google Searc x A www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQiHqRdYV 6Ux63Syp.JXz0Coxvwqgg4JkWI72X79QvOLp9_7U27sYQhkaocvdwecGvsUzo65uy3F6spORRg1XSqgh81is O STOICHIOMETRY Using molarity to find solute mass and solution volume Jacqueline A chemist adds 55.0 mL of a 4.75M silver perchlorate (AGCIO,) solution to a reaction flask. Calculate the mass in grams of silver perchlorate the chemist has added to the flask. Round your answer to 3 significant digits. Explanation Check Privacy Accessibil 2021 McGraw-Hill Education All Rights Reserved Terms of Usearrow_forwardd ← Chrome esc e 24% (92) ALE X Mathwa X G 102 cels X ☆ www-awu.aleks.com/alekscgi/x/isl.exe/1o_u-IgNsikr7j8P3jH-IvTqeviKFP6W0cqJcWJdIACROQwyw24GWHinMCnqLGstAPM iZtogOAPGJIWPnLIY... 17.4 Solubility and... 18.3 Gibbs Free E... 5.3 Enthalpies of... 18.5 Gibbs Free E... Reading Schedule 19.6 Reduction Po... Y SOLUTION: The le... Math 115 W-S Fall.... File email d x Edit View History Bookmarks Profiles View 7. Q A Nancy X Z Explanation O KINETICS AND EQUILIBRIUM Using the Arrhenius equation to calculate k at one temperature... 2 W S The rate constant of a certain reaction is known to obey the Arrhenius equation, and to have an activation energy E-29.0 kJ/mol. If the rate constant of this reaction is 2.8 x 10³ Ms at 320.0 °C, what will the rate constant be at 222.0 °C? -1 Round your answer to 2 significant digits. Check X A ALEKS X CIChego X H command # 3 E Tab Window Help D $ 4 C X R > F 5 (92) ALE X % 5 V I T G #tv Welcom X A ALEKS ^ 6 MacBook Pro B Y We 9 "/ & 7 H X U N You 8 J 0000015 1…arrow_forward= hrome File Edit View History Bookmarks Profiles Tab A ALEKS-Lara Althawadi -Lean X (129) ALEKS: Calculating the p X C www-awu.aleks.com/alekscgi/x/isl.exe/10_u-IgNsikr7j8P3jH-IvTqeviKFP6W0cqJcWJdIACROQwyw24GWHInwFNs98WNYISBfkoq97Td3eiTwdipb... 4 Solubility and O O ACIDS AND BASES Calculating the pH at equivalence of a titration esc Try Again Your answer is incorrect. 18.3 Gibbs Free E... 5.3 Enthalpies of... 18.5 Gibbs Free E... Reading Schedule 19.6 Reduction pH=9.22 Explanation 30 Methods for Measuring the pli of an Aqueous Slide 28 of 104 Notes A chemist titrates 160.0 mL of a 0.5839 M benzoic acid (HC,H,CO₂) solution with 0.4219M KOH solution at 25 °C. Calculate the pH at equivalence. The pK of benzoic acid is 4.20. Q Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of KOH solution added. A Recheck 2 Comments W S X Click to add movers 3 P: ---+63% E 5 D (129) ALEKS:…arrow_forward
- Learning AA prod03-cnow-owl.cengagenow.com Login Learning Learning × Online tea... y dr. marlow... ember L... with We 3. AS surroundi... M 2BrF3 (9) Br2 (g) + 3F2 (9) 4. AG° = AH°... M 5. AG: Pre... 1req 6. AG: Enthal... Using standard thermodynamic data at 298 K, calculate the free energy change when 2.14 moles of BrF3 (9) react at standard conditions. Substance AG (kJ/mol) 7. AG fro... 1req Question Question Question 8. Calculat... 1req AG° = 9. Calculat... 1req BrF3 (9) -229.4 Br2(g) 3.1 F2(9) 0.0 kjarrow_forward||| Calend ? College AL X ChatGP P College Q percen I Apply Gaseou Central Richard (387) u Ask a C + C www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-lgNslkr7j8P3jH-IBIZZxiNVNkWhorBIB0d5GFRhLZ5YclOUixZOSyCPbErWEdQp9Q4Y170EfAPQIj... Q □ G O Chemical Reactions Reaction sequence stoichiometry 0/5 Matthe Nitric acid is often manufactured from the atmospheric gases nitrogen and oxygen, and hydrogen prepared by reforming natural gas, in a two-step process. In the first step, nitrogen and hydrogen react to form ammonia: N2(g) + 3H2(g) → 2 NH, (g) In the second step, ammonia and oxygen react to form nitric acid (HNO3) and water: NH2(g) + 202(g) → HNO3(g) + H2O (g) Suppose the yield of the first step is 62.% and the yield of the second step is 87.%. Calculate the mass of hydrogen required to make 10.0 kg of nitric acid. Be sure your answer has a unit symbol, if needed, and is rounded to 2 significant digits. ☐ x10 Explanation Check OC 2024 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy…arrow_forwardDL IXL - Area and circur X E Hamilton w K Maya Paniagua - Sto x K Maya Paniagua - L10 X Stoichiometry Introd x amihq.com/web/viewer.html?state=%7B"ids"%3A%5B"1Jb_fUymErbX45jcXDbRuMhdNQC9zAIBE"%5D%2C*action"%3. Paniagua - Cl. 115% e A Kami Uploads Maya Paniagua - Stoichiometry WS1.pdf Chemistry Stoichiometry WS #1 Show all work!! No work = no credit! Per Date Name Mole to Mole Stoichiometry 1) For the reaction between magnesium fluoride and lead (IV) nitrate, there are 12 mol of magnesium fluoride available: a. Write and balance the chemical equation, indicate the type of reaction b. Find the mole amount of each species in the chemical reaction c. How many moles of the solid precipitate are formed?arrow_forward
- X A ALEKS-Allie Fleming - Knowled X + eks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQUHIQg6bJxmeSyVpHOEB1plef9xyC5Ca9Qlgg70JQ0BkW_S727IDRdmdWICDt8DxedZZpvVEqH-ZDXitxxGhUuDfQ-ZKhas?1 # * 3 E D E Module Knowledge Check Aqueous hydrobromic acid (HBr) reacts with solid sodium hydroxide (NaOH) to produce aqueous sodium bromide (NaBr) and liquid water (H₂O). What is the theoretical yield of water formed from the reaction of 2.4 g of hydrobromic acid and 0.48 g of sodium hydroxide? Round your answer to 2 significant figures. 08 I Don't Know Submit LIZ $ 4 R F 15 % 5 ▬▬ M 16 T G 0.9 6 Q Search X 17 4+ Y H & 7 Ś fa 144 U * fo 8 DII Question 5 - hp fo ( DDI 9 Al J K fm O () © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility L 112, insert P { prt sc [ با = () ← } 1 Allie V J BEEN delete olo Ar backspace h enarrow_forwardCH6_Chem103 - Kenai Peninsu X + to.mheducation.com/ext/map/index.html?_con=con&external_browser=0&launchUrl=https%253A%252F%252Fclasses.alaska.edu%252Fwebapps... 054 520 2 X 9 part 2 Homework i 47:54 J bok 50 O int rint erences C 125 aw 11 :0 FI 2 Question 11 - Chapter 9 part 2 X Determine the volume (in mL) of a 13.3 MHC2H3O2 stock solution must be used to prepare 200.0 mL of a solution that is 0.719 M HC2H302. F2 W mL HC2H302 # 3 00 20 F3 E $ 4 000 000 F4 R % 5 F5 U 8 DII F8 1 ( 9 DD F9 0 3 F10 Help P Savarrow_forwardomedion #i %23 eperalreaclimwrller os At28-X+20 üiithdred a yuelds e falloweing Lo's data' 0.150M 10.150M 0300M delerinne he in ale slC produdio (Aa JA 0,150M 0.300 3,20x10M dior(A[e]A A-0200M tha roarrow_forward
- nt X A ALEKS Bb in their s X Yuzu Re X U Labflow x O COVID- e Daily Cri X A Among X A www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-liJOkWvnm4w-aQ-rw-zRhgRnayfmbBs65spEliJ_PZNhkTHE Bb Blackboard O Mail - Ava Schied... O UAConnect Biology Syllabus U Labflow - Courses V Explore - HogSync ? PackE O CHEMICAL REACTIONS Solving for a reactant using a chemical equation Ammonia (NH3) chemically reacts with oxygen gas (02) to produce nitric oxide (NO) and water (H,O). What mass of nitric oxide is produced by the reaction of 4.8 g of oxygen gas? Round your answer to 2 significant digits. Explanation Check O 2020 McGraw-Hill E MacBook Proarrow_forwardraw The eneray dueram for ihis mechains m-racnan giv he PeaoNion ts endo thermit. Label all parts ľ , product, Acpi van on envray enhapy ntermediasks, acovatad Complex). Reactont Mechansm i Steja) Aaty -> N0 t 6,0 (sloxi). 切0十梅0 一ーはH(tor)arrow_forwardMoore 5e_C OWLV2 | Onli X Q ☆ * CE w.com/ilrn/takeAssignment/takeCovalentActivity... [Review Topics] [References] isms ots 2req Use the References to access important values if needed for this question. In a study of the conversion of methyl isonitrile to acetonitrile in the gas phase at 250 °C Visited 1 pts 2req CH3NC(g)- >CH3CN(g) 1 pts 2req the concentration of CH NC was followed as a function of time. It was found that a graph of In[CH3NC] versus time in seconds gave a 1 pts 2req straight line with a slope of -3.96x10-3 s and a y-intercept 52. 1 pts 2req Based on this plot, the reaction is v order in CH,NC and the rate constant for the reaction is 1 pts 2reg 1 pts 2reg 1 pts 2reg Submit Answer Retry Entire Group 9 more group attempts remaining Previous Next Email Instructor Save and Exit Cengage Learning | Cengage Technical Support 52% 1:41 PM 1/22/2021arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

