
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
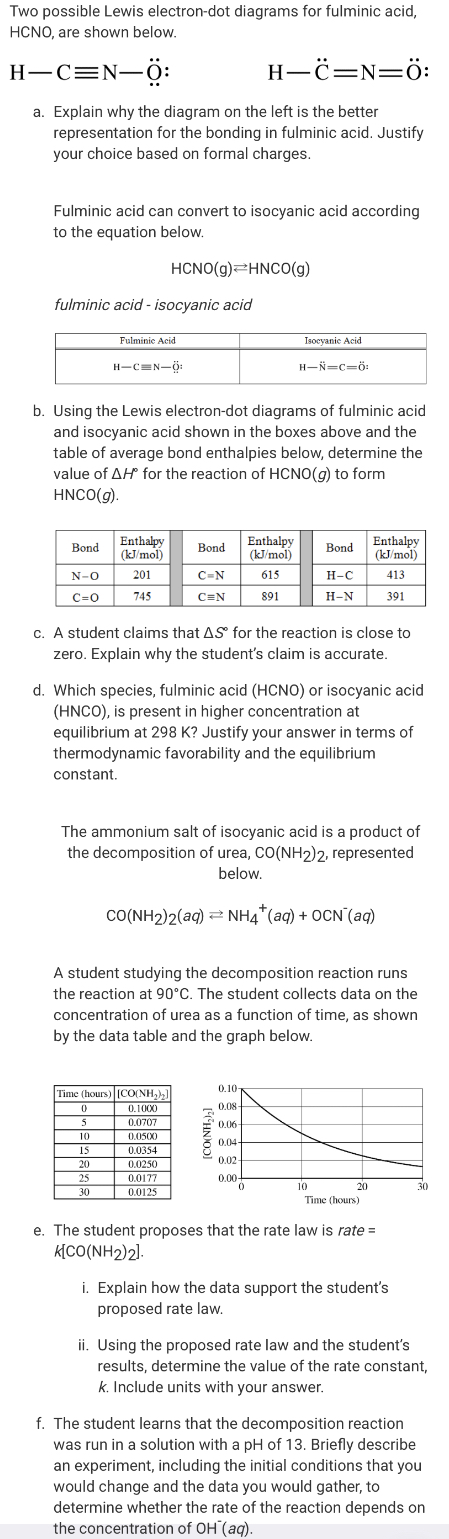
Transcribed Image Text:Two possible Lewis electron-dot diagrams for fulminic acid,
HCNO, are shown below.
Н—С%3DN—ӧ:
Н—ё—N—ӧ:
a. Explain why the diagram on the left is the better
representation for the bonding in fulminic acid. Justify
your choice based on formal charges.
Fulminic acid can convert to isocyanic acid according
to the equation below.
HCNO(g)=HNCO(g)
fulminic acid - isocyanic acid
Fulminic Acid
Isoeyanie Acid
н-сек-ф
н—й—с-б
b. Using the Lewis electron-dot diagrams of fulminic acid
and isocyanic acid shown in the boxes above and the
table of average bond enthalpies below, determine the
value of AH for the reaction of HCNO(g) to form
HNCO(g).
Enthalpy
(kJ/mol)
Enthalpy
(kJ/mol)
Enthalpy
(kJ/mol)
Bond
Bond
Bond
N-O
201
C-N
615
Н-С
413
c=0
745
C=N
891
Н-N
391
c. A student claims that AS for the reaction is close to
zero. Explain why the student's claim is accurate.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The value of Ka1 of hypophosphorous acid, H3PO2, is nearly the same as the Ka1 of phosphoric acid, H3PO4. Draw a Lewis structure for phosphoric acid that is consistent with this behavior. Draw the Lewis structure with the formal charges minimized. Do not include formal charges in your structure. Include hydrogen atoms and nonbonding electrons in the structure.arrow_forwardA similar chemical species is formed when Ba is in the presence of C and N, Ba(CN)2. Once again, all atoms obey the octet rule. Explain why you would say the type of bonding does not change .arrow_forwardBased on the bond energies for the reaction below, what is the enthalpy of the reaction? HC≡CH (g) + 5/2 O₂ (g) → 2 CO₂ (g) + H₂O (g)arrow_forward
- Can you please answer question 9.47 and all of the sub problems and show all of the steps to the solutionarrow_forwardAn incomplete Lewis structure is shown below. The structure only shows the atoms and how they are connected. The molecule has a net charge of +1. HH XX H H Complete the Lewis structure giving all atoms full octets. If there is more than one way to do this, draw resonance structures showing all possibilities. If not, just draw one Lewis structure. Be sure to write in any non-zero formal charges.arrow_forwardTwo possible Lewis structures for the cyanate ion are shown below, with formal charges (FC) calculated for each atom. One structure has carbon as the central atom and the other has a nitrogen as a central atom. By considering formal charges, which arrangement of atoms is the best, and therefore most likely structure for cyanate? FC: -1 0 0 FC: -1 +1 -1 |:0—C=N:1 STRUCTURE A STRUCTURE B Structure A Structure B Both are equally likely QUESTION 19 Two possible Lewis structures for the thiocyanate ion are shown below, with formal charges (FC) calculated for each atom. One structure has carbon as the central atom and the other has a nitrogen as a central atom. By considering formal charges, which arrangement of atoms is the best, and therefore most likely structure for thiocyanate? FC: 00-1 FC: -1 +1 -1 Ec: STRUCTURE A STRUCTURE B O Structure A Structure Barrow_forward
- An incomplete Lewis structure is shown below. The structure only shows the atoms and how they are connected. The molecule has a net charge of +1. Complete the Lewis structure giving all atoms full octets. If there is more than one way to do this, draw resonance structures showing all possibilities. If not, just draw one Lewis structure. Be sure to write in any non-zero formal charges.arrow_forwardWhat compound below has a central atom without an octet? OAICI3 O SiH4 O H2S O SiO2arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY