
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
Trend observed in graph and conclusion about the effect of temperature on enzyme activity.
i) include a concise description of the trend observed in the graph shown in question 3 above, and explain this trend using the language presented in this unit and your biochemical knowledge of enzymes and reactions. In your conclusion, provide a logical argument supported by molecular theory that would explain any change observed in enzyme activity.
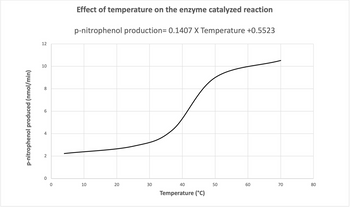
Transcribed Image Text:p-nitrophenol produced (nmol/min)
12
10
8
6
4
2
0
0
Effect of temperature on the enzyme catalyzed reaction
p-nitrophenol production= 0.1407 X Temperature +0.5523
10
20
30
40
Temperature (°C)
50
60
70
80
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Similar questions
- What effects does pH have on enzyme activity? Cite relevant experimental data. How can you explain this relationship(cause)?arrow_forwardCan you please help which answers if fitting for lowering the activation energy?arrow_forwardPlease Explain each part to the best of your ability. This is Biochemistry. Thank youarrow_forward
- Enzyme activity regulation by other molecules ( must include role of inhibitors, allosteric site and activators )arrow_forwardEnzyme Activity and Physiological Function, The Vmax of the enzyme glycogen phosphorylase from skeletal muscle is much greater than the Vmax of the same enzyme from liver tissue. (a) What is the physiological function of glycogen phosphorylase in skeletal muscle?arrow_forwardGive detailed Solution with explanation needed (don't give Handwritten answerarrow_forward
- Matching (All the highlighted answers are wrong please explain why it is wrong and give me the correct answer. Thanks, in advanced)arrow_forwardANSWER A AND B PLSarrow_forwardThis is the kcat question. At maximum saturation, 28.3µg of enzyme in 25mL water catalyzes the oxidation of ethanol at a rate of 2.5mM/min. Calculate kcat in units of seconds if the enzyme has a molar mass of 65kg/mol. The more correct work you show, the more credit you will get.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON