
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
To measure the amount of citric acid (CzHgO(CO,#/)g) in a certain candy, an analytical chemist dissolves a 21.00 g sample of the candy in 200. mL. of water
and titrates this solution to the endpoint with 12.8 mL of 0.280 M sodium hydroxide (NaH) solution,
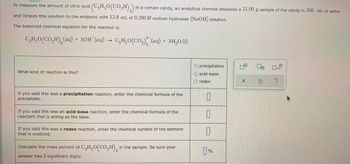
Transcribed Image Text:To measure the amount of citric acid
and titrates this solution to the endpoint with 12.8 mL of 0.280 M sodium hydroxide (NaOH) solution.
The balanced chemical equation for the reaction is:
CyH5O(CO,H),(aq) +30H (aq) C₂H₂O(CO₂) (aq) + 3H₂0 (1)
(C3H5O(CO,H),) in a certain candy, an analytical chemist dissolves a 21.00 g sample of the candy in 200. mL of water
What kind of reaction is this?
If you said this was a precipitation reaction, enter the chemical formula of the
precipitate.
If you said this was an acid-base reaction, enter the chemical formula of the
reactant that is acting as the base.
If you said this was a redox reaction, enter the chemical symbol of the element
that is oxidized.
Calculate the mass percent of C3H₂O(CO₂H), in
in the sample. Be sure your
answer has 3 significant digits.
O precipitation
O acid-base
O redox
0
0
0
0%
X
L
S
x10
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- By titration, it is found that 19.1 mL of 0.157 M NaOH(aq) is needed to neutralize 25.0 mL of HCl(aq). Calculate the concentration of the HCI solution.arrow_forwarding e Volume of base needed to titrate a given mass... A chemistry student weighs out 0.196 g of citric acid (H,C H¸0,), a triprotic acid, into a 250. mL volumetric flask and dilutes to the mark with distilled 6**5 water. He plans to titrate the acid with 0.0600 M NaOH solution. Calculate the volume of NaOH solution the student will need to add to reach the final equivalence point. Round your answer to 3 significant digits. I mL x10 の Explanation Check © 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use l Privacy Acce 7:49 PM ^ O 4) ENG P Type here to search 2/27/2021 DELL PrtScr Insert Home End Esc F8 F9 F10 F11 F12 F1 F2 F3 F4 F5 F6 F7 Fn # 24 & 6 1 7 Y 8 A 9 9 0arrow_forwardA solution of NaCl(aq) is added slowly to a solution of lead nitrate, Pb(NO,),(aq), until no further precipitation occurs. The precipitate is collected by filtration, dried, and weighed. A total of 15.67 g PbCl, (s) is obtained from 200.0 mL of the original solution. Calculate the molarity of the Pb(NO,),(aq) solution. concentration: Marrow_forward
- a student weights out 1.118 g of impure KHP, dissolves the sample in deionized water and titrates it with 0.1001 M NaOH solution. If the titration requires 2710 mL of the NaOH solution, and non of the impurities react with NaOH, what is the percent KHP in the sample?arrow_forwardA student weighs out a 3.48 g sample of manganese(II) chloride, transfers it to a 125 mL volumetric flask, adds enough water to dissolve it and then adds water to the 125 mL tic mark. What is the molarity of MnCl, in the resulting solution?arrow_forwardPlease don't provide handwritten solution ...arrow_forward
- All the chloride in a 5.00 g sample of an unknown metal chloride is precipitated as AgCl with 70.9 mL of 0.2010 M AgNO3. What is the percentage of chloride in the sample?arrow_forwardCalculate the volume of NaOH solution the student will need to add to reach the final equivalence point. Be sure your answer has the correct number of significant digits.arrow_forward5. A student conducted a titration experiment. He/she used 12.0 mL of silver nitrate (titrant) solution to reach the endpoint. If the student used 25.4 mL of the chloride (analyte) for the titration and found the concentration of chloride ions to be 2.2 M, what is the mass (in grams) of silver nitrate titrant Clic)arrow_forward
- b) Water "softeners" work via the principle of ion-exchange. The tank of a water softener is filled with a soluble salt such as sodium chloride. As "hard" water passes through the tank, the undesirable ions in hard water (e.g., calcium, magnesium, and iron III) are exchanged for the more desirable "soft" (meaning more soluble) ions like sodium. Explain how swapping the "soft"-water sodium ions for "hard"-water ions would influence the behavior (the cleaning effectiveness) of soaps and detergents.arrow_forwardA 185.0 mL sample of 1.200 M Pb(NO₃)₂ is mixed with 97.50 mL of 1.500 M NaCl, and the PbCl₂ precipitate is filtered from the solution. Then 200.0 mL of 3.000 M NaBr is added to the remaining solution, and the PbBr₂ precipitate is also collected and dried. What is the mass (in grams) of the PbBr₂ precipitate, assuming the yield in each precipitation step is 100%?arrow_forwardA chemistry student needs to standardize a fresh solution of sodium hydroxide. He carefully weighs out 79. mg of oxalic acid (H,C,0,), a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in 250. mL of distilled water. The student then titrates the oxalic acid solution with his sodium hydroxide solution. When the titration reaches the equivalence point, the student finds he has used 23.0 mL of sodium hydroxide solution. Calculate the molarity of the student's sodium hydroxide solution. Be sure your answer has the correct number of significant digits.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY