
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
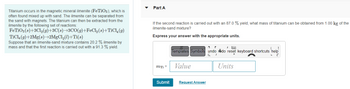
Transcribed Image Text:Titanium occurs in the magnetic mineral ilmenite (FeTiO3), which is
often found mixed up with sand. The ilmenite can be separated from
the sand with magnets. The titanium can then be extracted from the
ilmenite by the following set of reactions:
FeTiO3(s)+3Cl₂(g)+3C(s)→3CO(g)+FeCl₂
TiCl4 (g)+2Mg(s)→2MgCl₂(1)+Ti(s)
(s)+TiCl₂(g)
Suppose that an ilmenite-sand mixture contains 20.2 % ilmenite by
mass and that the first reaction is carried out with a 91.3 % yield.
Part A
If the second reaction is carried out with an 87.0 % yield, what mass of titanium can be obtained from 1.00 kg of the
ilmenite-sand mixture?
Express your answer with the appropriate units.
mTi =
Submit
1 r
Templates Symbols undo redo reset keyboard shortcuts
¼/
/
Value
Request Answer
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following statements is true for the following reaction, assuming the given reaction proceeds in the forward direction? Fe*(aq) + Co(s) → Fe2*(aq) + Co2*(aq) A Fe3*(aq) is oxidized and Co(s) is reduced. Fe*(aq) is oxidized and Co2*(aq) is reduced. Co(s) is oxidized and Fe3*(aq) is reduced. Co(s) is oxidized and Co2*(aq) is reduced. E Fe2*(aq) is oxidized and Co(s) is reduced.arrow_forwardWrite a balanced chemical equation for the standard formation reaction of solid vanadium(V) oxide (V,05).arrow_forward2 V2O5 + 5 Si → 4 V + 5 SiO2 Given the above balanced equation, calculate the number of grams of silicon required to prepare 307 grams of vanadium metal.arrow_forward
- The thermite reaction is written below. Show that the heat released in this reaction is sufficient for the iron to be produced as molten metal. Find the enthalpy of formation of the overall reaction. Al(s) + Where: Al ΔΗ°F= 0 Al2O3 AHF -2076 Fe AHF 0 Fe2O3 AHF -745 Fe₂O3(s)- → Al₂O3(s) + Fe(1)arrow_forwardA sample of steel was dissolved and the manganese present was converted to MnO4– (aq). Water was then added to give a final solution volume of 100.00 mL. A sample of this solution was examined and was found to have an absorbance at 525 nm of 0.560 (pathlength = exactly 1 cm). What is the concentration of MnO4– in the solution? (The molar absorptivity is 3.62 × 103 L/(mol cm).)arrow_forwardSodium hydrogen sulfate is used as a cleaning agent and as a flux (a substance that promotes the fusing of metals and prevents the formation of oxides). One of the ways in which sodium hydrogen sulfate is manufactured is by reacting sodium dichromate, Na2Cr2O7, with sulfuric acid. This process also forms water and chromium(VI) oxide, CrO3. Write a balanced equation for this reaction. (You do not need to include states.) How many kilograms of sodium dichromate, Na2Cr2O7, are necessary to produce 130.4 kg of sodium hydrogen sulfate? How many kilograms of chromium(VI) oxide are formed when 130.4 kg of sodium hydrogen sulfate is made? What is the minimum volume of 18.0 M H2SO4 solution necessary to react with 874.0 kg of sodium dichromate? What is the maximum mass of sodium hydrogen sulfate, NaHSO4, that can be formed from the reaction of 874.0 kg of sodium dichromate with 400.0 L of 18.0 M H2SO4?arrow_forward
- What is the struture of this product and what type of reaction is this?arrow_forwardFine particles of metallic iron can be injected underground to remediate pollution of underground aquifers by the industrial solvent trichloroethane. In one experiment, 2400L of an aqueous emulsion containing ~480 kg of Fe(0) consumed 17.0 kg of trichloroethane in 5 months. Write a balanced reaction using H2O and H+ to complete the balancing. What percentage of injected iron was used by this reaction in 5 months? Fe + C2HCl3 ---> Fe2+ + C2H4 + Cl-arrow_forwardCalculate the equilibrium constant for the reaction: NO2 (g) + NO (g) N2O (g) + O2 (g)arrow_forward
- The Fe2+Fe2+ (55.84555.845 g/mol) content of a 2.2472.247 g steel sample dissolved in 50.0050.00 mL of an acidic solution was determined by tiration with a standardized 0.1200.120 M potassium permanganate (KMnO4KMnO4, 158.034158.034 g/mol) solution. The titration required 29.2929.29 mL to reach the end point. What is the concentration of iron in the steel sample? Express your answer as grams of FeFe per gram of steel. MnO−4+8H++5Fe2+↽−−⇀Mn2++5Fe3++4H2OMnO4−+8H++5Fe2+↽−−⇀Mn2++5Fe3++4H2O concentration: g Fe/g steel (please type answer not write by hend)arrow_forwardGiven Zn(s) and AL(s), what observations would you make when combining AL(s) with ZnSO4(aq), and combining Zn(s) with Al(SO4)3(aq)?arrow_forwardKk.297.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY