
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
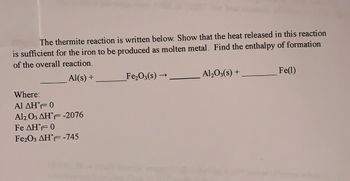
Transcribed Image Text:The thermite reaction is written below. Show that the heat released in this reaction
is sufficient for the iron to be produced as molten metal. Find the enthalpy of formation
of the overall reaction.
Al(s) +
Where:
Al ΔΗ°F= 0
Al2O3 AHF -2076
Fe AHF 0
Fe2O3 AHF -745
Fe₂O3(s)-
→
Al₂O3(s) +
Fe(1)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Given that: AH°F [Fe(OH)3 (s)] -- 823.0 kJ/mol AH°F [Fe+3 (aq)] = - 48.5 kJ/mol AH°F [OH (aq)] = - 230.0 kJ/mol What is the enthalpy change for the precipitation reaction of Fe(OH)3 (s)? O A-1101.5 kJ/mol O B.- 84.5 kJ/mol Oc-447.5 kJ/mo1 O D.- 1561.5 kJ/mol O E. - 544.5 kJ/molarrow_forwardA student dissolves 11.5 g of ammonium nitrate (NH4NO3) in 200. g of water in a well-insulated open cup. She then observes the temperature of the water fall from 20.0 °C to 16.6 °C over the course of 4 minutes. Use this data, and any information you need from the ALEKS Data resource, to answer the questions below about this reaction: NH4NO3(s) NH(aq) + NO, (aq) You can make any reasonable assumptions about the physical properties of the solution. Be sure answers you calculate using measured data are rounded to the correct number of significant digits. Note for advanced students: it's possible the student did not do the experiment carefully, and the values you calculate may not be the same as the known and published values for this reaction. Is this reaction exothermic, endothermic, or neither? If you said the reaction was exothermic or endothermic, calculate the amount of heat that was released or absorbed by the reaction in this case. Calculate the reaction enthalpy ΔΗ AH per mole of…arrow_forwardA student dissolves 14.0 g of ammonium nitrate (NH4NO3) in 200. g of water in a well-insulated open cup. He then observes the temperature of the water fall from 22.0 °C to 16.6 °C over the course of 5.5 minutes. Use this data, and any information you need from the ALEKS Data resource, to answer the questions below about this reaction: NH4NO₂ (s) NH(aq) + NO3(aq) - You can make any reasonable assumptions about the physical properties of the solution. Be sure answers you calculate using measured data are rounded to the correct number of significant digits. Note for advanced students: it's possible the student did not do the experiment carefully, and the values you calculate may not be the same as the known and published values for this reaction. Is this reaction exothermic, endothermic, or neither? If you said the reaction was exothermic or endothermic, calculate the amount of heat that was released or absorbed by the reaction in this case. Calculate the reaction enthalpy AHxn per mole…arrow_forward
- Item 22 22 of 25 I Review I Constants I Periodic Table Classify the following as exothermic or endothermic reactions and give AH for each. Part E Classify reaction as exothermic or endothermic. Formation of aluminum oxide and copper from aluminum and copper (II) oxide: 2Al(s)+3CuO(s)→Al2O3(s)+3Cu(s)+1204kJ exothermic reaction O endothermic reaction Part F Give AH for reaction above. Express your answer as an integer. ? kJarrow_forwardThe Sun supplies about 1.0 kilowatt of energy for each squaremeter of surface area (1.0 kW/m2, where a watt = 1 J/s).Plants produce the equivalent of about 0.20 g of sucrose(C12H22O11) per hour per square meter. Assuming that thesucrose is produced as follows, calculate the percentage ofsunlight used to produce sucrose.12 CO2(g)+ 11 H2O(l)-----> C12H22O11 + 12 O2(g)ΔH = 5645 kJarrow_forward1. How much energy in kJ is released when 124.49 g of nickel react with an excess of phosphorus? 5 Ni + 2 P -> Ni5P2 △ H = 436 kJ/mol 2.How much titanium in grams is required to release 538.13 kJ of heat? Ti + 2 B -> TiB2 △ H = 316 kJ/molarrow_forward
- Give detailed Solutionarrow_forwardGiven the overall reaction from the previous problem: 2 CO (g) + 6 H2 (g) D 2 CH4 (g) + 2 H2O (g) Using Le Chatelier’s principle, predict which direction the reaction will shift to reestablish equilibrium for each of the following changes: Calculate the enthalpy of reaction for this reaction using the standard thermodynamics values for the reactants and products. Is the reaction endothermic or exothermic? Now, use Le Chatelier’s principle again to determine the direction the equilibrium will shift if heat is added to the reaction. If 35 kPa of CO is mixed with 35 kPa of hydrogen in a 1.5 L container at 25°C, what is the theoretical yield of methane?arrow_forwardExplain step by step why each can or cannot occur.arrow_forward
- 1. How many kJ of energy are released by 34.65 g of boron? Ti + 2 B -> TiB2 △ H = 316 kJ/mol 2. How much energy in kJ is released when 14.17 g of aluminum react with an excess of copper oxide? 3 CuO + 2 Al -> 3 Cu + Al2O3 △ H = 1190 kJ/mol 3. If 36.26 grams of aluminum is heated from 18 oC to 26 oC how much energy was required? sAl = 0.897 J/(gK)arrow_forwardFor a reaction with ΔHo = 40 kJ/mol, decide which of the following statements is (are) true. Correct any false statement to make it true. (a) The reaction is exothermic; (b) ΔGo for the reaction is positive; (c) Keq is greater than 1; (d) the bonds in the starting materials are stronger than the bonds in the product; and (e) the product is favored at equilibrium.arrow_forwardThe reaction of CH3OH(g) with N2(g) to give HCN(g), NH3(g), and O2(g) requires 164 kJ/mol of CH3OH(g). Write a balanced chemical equation for this reaction. Include the phases of all species in the reaction. How much heat is involved in the reaction of 65.0 g of CH3OH(g) with excess N2(g) to give HCN(g) and NH3(g) in this reaction?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY