
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Transcribed Image Text:部
23
K=2
At equilibrium?
K
At equilibrium?
○ yes
O no
○ yes
○ no
○ yes
O no
○ yes
O no
K=1
At equilibrium?
K=1
At equilibrium?

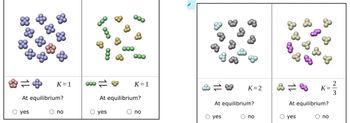
Transcribed Image Text:Tiny samples of aqueous solutions are sketched below, as if under a microscope so powerful that individual molecules could be seen. (The water molecules are
not shown.)
The two substances in each sample can interconvert. That is, each kind of molecule can turn into the other. The equilibrium constant K for each interconversion
equilibrium is shown below the sketch.
Decide whether each solution is at equilibrium.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Explain that equilibrium is dynamic, and that at equilibrium the forward and backward reaction rates are equal.arrow_forwardWhat is the law of mass action? Is it true that the value of K depends on the amounts of reactants and products mixed together initially? Explain. Is it true that reactions with large equilibrium constant values are very fast? Explain. There is only one value of the equilibrium constant for a particular system at a particular temperature, but there is an infinite number of equilibrium positions. Explain.arrow_forwardConsider 0.200 mol phosphorus pentachloride sealed in a 2.0-L container at 620 K. The equilibrium constant, Kc, is 0.60 for PCl5(g) PCl3(g) + Cl2(g) Calculate the concentrations of all species after equilibrium has been reached.arrow_forward
- In Section 13.1 of your text, it is mentioned that equilibrium is reached in a closed system. What is meant by the term closed system. and why is it necessary to have a closed system in order for a system to reach equilibrium? Explain why equilibrium is not reached in an open system.arrow_forwardBecause calcium carbonate is a sink for CO32- in a lake, the student in Exercise 12.39 decides to go a step further and examine the equilibrium between carbonate ion and CaCOj. The reaction is Ca2+(aq) + COj2_(aq) ** CaCO,(s) The equilibrium constant for this reaction is 2.1 X 10*. If the initial calcium ion concentration is 0.02 AI and the carbonate concentration is 0.03 AI, what are the equilibrium concentrations of the ions? A student is simulating the carbonic acid—hydrogen carbonate equilibrium in a lake: H2COj(aq) H+(aq) + HCO}‘(aq) K = 4.4 X 10"7 She starts with 0.1000 AI carbonic acid. What are the concentrations of all species at equilibrium?arrow_forward. What does it mean to say that a state of chemical or physical equilibrium is dynamic?arrow_forward
- Describe a nonchemical system that is in equilibrium, and explain how the principles of equilibrium apply to the system.arrow_forward12.101 An engineer working on a design to extract petroleum from a deep thermal reservoir wishes to capture toxic hydrogen sulfide gases present by reaction with aqueous iron(II) nitrate to form solid iron(II) sulfide. (a) Write the chemical equation for this process, assuming that it reaches equilibrium. (b) What is the equilibrium constant expression for this system? (c) How can the process be manipulated so that it does not reach equilibrium, allowing the continuous removal of hydrogen sulfide?arrow_forwardWrite equilibrium constant expressions for the following generalized reactions. a. 2X(g)+3Y(g)2Z(g) b. 2X(g)+3Y(s)2Z(g) c. 2X(s)+3Y(s)2Z(g) d. 2X(g)+3Y(g)2Z(s)arrow_forward
- In Section 17.3 of your text, it is mentioned that equilibrium is reached in a closed system. What is meant by the term “closed system,” and why is it necessary for a system to reach equilibrium? Explain why equilibrium is not reached in an open system.arrow_forwardA solution is prepared by dissolving 0.050 mol of diiodocyclohexane, C5H10I2, in the solvent CCl4.The total solution volume is 1.00 L When the reaction C6H10I2 C6H10 + I2 has come to equilibrium at 35 C, the concentration of I2 is 0.035 mol/L. (a) What are the concentrations of C6H10I2 and C6H10 at equilibrium? (b) Calculate Kc, the equilibrium constant.arrow_forward5.2. What is the difference between a static equilibrium and a dynamic equilibrium? Give examples difference from the examples in the text. What is similar for the two types of equilibria?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning