
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please correct answer and don't use hand rating
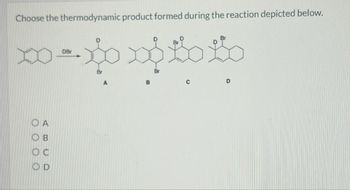
Transcribed Image Text:Choose the thermodynamic product formed during the reaction depicted below.
DBr
ABCD
OA
OD
Br
A
B
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 5 images

Knowledge Booster
Similar questions
- What characterizes an electrolytic cell? What is an ampere? When the current applied to an electrolytic cell is multiplied by the time in seconds, what quantity is determined? How is this quantity converted to moles of electrons required? How are moles of electrons required converted to moles of metal plated out? What does plating mean? How do you predict the cathode and the anode half-reactions in an electrolytic cell? Why is the electrolysis of molten salts much easier to predict in terms of what occurs at the anode and cathode than the electrolysis of aqueous dissolved salts? What is overvoltage?arrow_forwardCobalt metal can be prepared by reducing cobalt (II) oxide with carbon monoxide. CoO(s)+CO(g)Co(s)+CO2(g)Kc=4.90102 at 550 Carrow_forwardChoose the thermodynamic product formed during the reaction depicted below. DBr OA B OC OD Br Br Br A B Carrow_forward
- H2N. Br CI H2N. Br CI H2N. Br CIarrow_forwardCan anyone explain how this reaction happens clearlyarrow_forwardPhosphoric and acetic acids are considered to be weak acids; that is, they do not dissociate completely into ions. Were there any differences in reaction rates with magnesium between these two acids? If so, what distinguishing feature of the acids might explain the differences?arrow_forward
- Breathalyzers measure the alcohol (ethanol, C2H6O) content in an exhaled breath using a redox reaction. The C2H6O reacts with an acidic solution of potassium dichromate, forming acetic acid. The color of the solution changesbecause some of the orange chromate is converted to the green Cr3+, and the Breathalyzer measures this color change.C2H6O + Cr2O7 = C2H4O2 + Cr3+ (UNBALANCED)(a) Which reactant is oxidized? Which reactant is reduced?Which reactant is the oxidizing agent? Which reactant is the reducing agent?(b) Balance the redox reaction above that occurs in a Breathalyzer. Note the reaction occurs in acidic conditions.(c) What is E° for the reaction if the standard reduction potential for the acetic acid / ethanol half-reaction is 0.058 V?(Note: see Appendix L in the OpenStax textbook for the reduction potential of the other half-reaction)arrow_forwardComplete this table for the reaction T(k) [N2] [H2] [NH3] [Kc] 500 0.115 0.105 0.439 575 0.110 0.128 9.6 At which temperature is the product favored the most?arrow_forwardPredict the product for the following reaction. || excess H₂ Ni, 100 atm, 150°C IV HO Varrow_forward
- What is the thermodynamic product of this reaction? HBr Br O Br Br O Br Br Brarrow_forwardPlease explain step by step.arrow_forwardHere is a more complex redox reaction involving the dichromate ion in acidic solution: 3S2− + 14H+ + Cr2O72−→ 3S +2Cr3+ + 7H2O Classify each reactant as the reducing agent, oxidizing agent, or neither. (H+, S2-,Cr2O72−)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning