
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
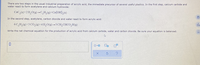
Transcribed Image Text:There are two steps in the usual industrial preparation of acrylic acid, the.immediate precursor of several useful plastics. In the first step, calcium carbide and
water react to form acetylene and calcium hydroxide:
CaC,(s)+2 H,O(g)→C,H,(9)+Ca(OH),(s)
In the second step, acetylene, carbon dioxide and water react to form acrylic acid:
6C,H,(9)+3 CO,(g)+4 H,O(9)→5 CH,CHCO,H(9)
dla
Write the net chemical equation for the production of acrylic acid from calcium carbide, water and carbon dioxide. Be sure your equation is balanced.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Propane can be turned into hydrogen by the two-step reforming process. In the first step, propane and water react to form carbon monoxide and hydrogen: C3H3(9)+3 H,O(g)→3 CO(g)+7H,(g) In the second step, carbon monoxide and water react to form hydrogen and carbon dioxide: CO()+H,O(g)→H(9)+CO,(g) Write the net chemical equation for the production of hydrogen from propane and water. Be sure your equation is balanced. O-0 Check Explanation 02021 McGraw Hill LLC. All Rights Reserved. Terms of Use Pivacy Center 日のarrow_forwardThe extraction of aluminum metal from the aluminum hydroxide in bauxite ore by the Hall-Héroult process is one of the most remarkable success stories of late 19th century practical chemistry, turning aluminum from a rare and precious metal into the cheap commodity it is today. In the first step, aluminum hydroxide reacts to form alumina and water: 2 Al(OH)3(s)-Al₂O3(s)+3 H₂O(g) In the second step, alumina and carbon react to form aluminum and carbon dioxide: 2 Al₂O3(s)+3 C(s)-4 Al(s) + 3 CO₂(g) do Write the net chemical equation for the production of aluminum from aluminum hydroxide and carbon. Be sure your equation is balanced. X Ś ?arrow_forwardV There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. In the first step, calcium carbide water react to form acetylene and calcium hydroxide: CaC₂(s) + 2 H₂O(g) → C₂H₂(9) + Ca(OH)₂(s) In the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6C₂H₂(g) + 3 CO₂(g) + 4H₂O(g) → 5 CH₂CHCO₂(g) Calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. Round your answer to the nearest kJ. 0 kJ esc Explar Explanation Check X ΔΗ= -414. kJ > Costa ΔΗ=132. kJ MacBook Pro © 2022 McGraw Hill LLC. All Rights Reserved. You Terms of Use | Privacy Center |arrow_forward
- Your lab partner accidentally mixed some sodium chloride with your sample of Epsom salts (MgSO₄・7H₂O). You want to make a standard solution of magnesium ion, and this is the only sample of a magnesium salt you have. To determine the amount of magnesium salt in the mixture, you heat 100.00 g to drive off the water of hydration and find that the anhydrous mixture has a mass of 55.25 g. What is the mass in grams of the original salt mixture that you must add to 1.000 L of water to make a 0.1000 M solution of Mg²⁺ ion?arrow_forward8.) Incomplete combustion of hydrocarbons leads to the generation of carbon monoxide rather than carbon dioxide. As a result, improperly vented furnaces can poison people who live in an affected building because of the toxicity of CO. Calculate the heat of reaction for the incomplete combustion of methane, CH4(g), to yield liquid water and CO(g).arrow_forwardConsider the reaction for the production of NO₂ from NO: 2 NO(g) + O₂ (g) → 2 NO₂ (g)arrow_forward
- Heptacarbon octahydride is an effective gasoline additive. In the presence of oxygen gas (and a spark), it undergoes complete combustion. (c) Calculate the mass of oxygen gas necessary to produce a theoretical yield of 1.00 lb of carbon dioxide.arrow_forwardConsider the reaction below for the combustion of methane (CH4, which is the principal component of natural gas). When natural gas is combusted, heat is given off that can be used to provide warmth in homes as well as to heat water, and it is increasingly used as an alternative to coal for the production of electricity. CH4(g) + 2O2(g) → CO2(g) + 2H2O(g) + q Which of the following statements are correct? The combustion of methane is endothermic, and the sign of ∆Hrxn is negative. The combustion of methane is endothermic, and the sign of ∆Hrxn is positive. The combustion of methane is exothermic, and the sign of ∆Hrxn is positive. The combustion of methane is exothermic, and the sign of ∆Hrxn is negative.arrow_forwardBalance the following chemical equation (if necessary):arrow_forward
- Which of the following is the formation reaction for Na2CO3 (s)? A. 2 Na(s) + C(s, graphite) + 3 O(g) → Na2CO3(s) B. 4 Na(s) + 2 C(s, graphite) + 3 O2(g) → 2 Na2CO3(s) C. 2 Na(s) + C(s, graphite) + 1.5 O2(g) → Na2CO3(s) D. Na2(s) + C(s, graphite) + 1.5 O2(g) → Na2CO3(s) E. 2 Na(g) + C(g) + 1.5 O2(g) → Na2CO3(s)arrow_forwardThere are two steps in the extraction of copper metal from chalcocite, a copper ore. In the first step, copper(I) sulfide and oxygen react to form copper(I) oxide and sulfur dioxide: 2Cu,S(s) + 30,(g) - 2Cu,O(s) + 2SO, (g) In the second step, copper(I) oxide and carbon react to form copper and carbon monoxide: Cu,O(s) + C(s) → 2Cu(s) + CO (g) ol Suppose the yield of the first step is 82.% and the yield of the second step is 83.%. Calculate the mass of oxygen required to make 1.0 kg of copper. Be sure your answer has a unit symbol, if needed, and is rounded to 2 significant digits. x10arrow_forward8. A 1.107-g sample of benzoic acid (HC;H;O2) is burned in an excess of O:(g) in a bomb calorimeter, which is immersed in water. The reaction produces heat that causes the temperature of the water and bomb to increase from 24.96 to 29.20°C. (a) If the heat capacity of the calorimeter is 6.90 kJ/ºC, how many kilojoules of heat are produced by the combustion of 1.107 g of benzoic acid? (b) Calculate the heat of combustion per gram of benzoic acid and the molar enthalpy of combustion of benzoic acid.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY