
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
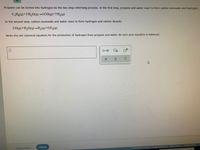
Transcribed Image Text:Propane can be turned into hydrogen by the two-step reforming process. In the first step, propane and water react to form carbon monoxide and hydrogen:
C3H3(9)+3 H,O(g)→3 CO(g)+7H,(g)
In the second step, carbon monoxide and water react to form hydrogen and carbon dioxide:
CO()+H,O(g)→H(9)+CO,(g)
Write the net chemical equation for the production of hydrogen from propane and water. Be sure your equation is balanced.
O-0
Check
Explanation
02021 McGraw Hill LLC. All Rights Reserved. Terms of Use Pivacy Center
日の
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Explain why each of the following chemical equations is not a correct formation reaction:(a) 4 AI(s) + 3 O2(g)→2 AI2O3(s)(b) N2(g) +3/2 H2(g) → NH3(g)(c) 2 Na(s) + O(g) → Na2O(s)arrow_forwardThe reaction that was on the screen when you started and its derivative demonstrate that the reaction enthalpy, ΔH, changes sign when a process is reversed. Consider the reaction H2O(l)→H2O(g), ΔH =44.0kJ What will ΔH be for the reaction if it is reversed?arrow_forwardO = rosoft C W There are two steps in the extraction of copper metal from chalcocite, a copper ore. In the first step, copper(I) sulfide and oxygen react to form copper(1) oxide and sulfur dioxide: 2Cu₂S (s) + 30₂(g) → 2Cu₂0(s) + 2 SO₂ (g) In the second step, copper(I) oxide and carbon react to form copper and carbon monoxide: Cu₂O(s) + C(s)→ 2Cu(s) + CO (g) Microsoft O CHEMICAL REACTIONS Reaction sequence stoichiometry Microsoft 6.52.210... esc Suppose the yield of the first step is 64.% and the yield of the second step is 88.%. Calculate the mass of oxygen required to make 2.0 kg of copper. Be sure your answer has a unit symbol, if needed, and is rounded to the correct number of significant digits. Explanation aption Q A T N 11 2 Check 9,039 W S X command #3 E 010 14 4 X $ C DEC 3 R F Ś % 5 V T útv G () > Y Ø 7 H Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibil 917 C N * 00 8 J FERBARKE I A M -0 K O L 28 W commandarrow_forward
- Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. In the first step, nitrogen and hydrogen react to form ammonia: N,(0) + 3 H,(9) → 2 NH,(9) ΔΗ-92. k In the second step, ammonia and oxygen react to form nitric acid and water: NH,(9) + 20,(9) → HNO3(g) + H,O(g) AH=-330. kJ Calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. Round your answer to the nearest kJ. ?arrow_forwardThe reaction between nitrogen and oxygen is given below: N2(g) + O2(g) → 2 NO(g) We therefore know that which of the following reactions can also occur? | 2 NO2(g) → 2 NO(g) + O2(g) 2 NO(g) → N2(g) + O2(g) 2 NO(g) + O2(g)→ 2 NO2(g) O None of the Abovearrow_forwardConsider the synthesis of ammonia: N2 (g) +3H, (g) 2NH3 (g)arrow_forward
- 1) When HCl(aq) and NaOH(aq) are mixed in a beaker, the beaker feels warm to the touch. What is known about the enthalpy of this reaction? The reaction is exothermic. ΔH is positive. The reaction is endothermic. Heat is absorbed from the surroundings. 2) Consider this combination reaction:2Mg(s)+O2(g)→2MgO(s) ΔH=−1204 kJ What is the enthalpy for the decomposition of 1 mole of MgO(s) into Mg(s) and O2(g)? -602 kJ/mol 602 kJ/mol -1204 kJ/mol 1204 kJ/mol 3) The enthalpy for the formation of 1 mole of NH3(aq) is -80.29 kJ/mol. What is the enthapy for the formation of 3 moles of NH3(aq)? -240.87 kJ −518×103 kJ -26.76 kJ -83.29 kJarrow_forward1arrow_forwardFor each of the following reactions, draw the enthalpy diagram showing the relative energy state of reactants and products. i). SO2 (g) → S(s)+ O2 (g) ΔH = +296 kJ ii). 2 C2H2 (g) + 5 O2 (g) → 4 CO2 (g) + 2 H2O(l) ΔH = -2598.8 kJarrow_forward
- I know you this question has two parts but it counts as one question. Thank you so much and I hope you have a nice day.arrow_forwardDimethyl ether, a useful organic solvent, is prepared in two steps. In the first step, carbon dioxide and hydrogen react to form methanol and water: CO₂(g) + 3 H₂(g) CH₂OH(1) + H₂O(1) In the second step, methanol reacts to form dimethyl ether and water: 2 CH₂OH(1)→ CH₂OCH3(g) + H₂O(1) Calculate the net change in enthalpy for the formation of one mole of dimethyl ether from carbon dioxide and hydrogen from these reactions. Round your answer to the nearest kJ. เม kJ AH=-131. kJ Ś ΔΗ= 8. kJarrow_forward3. Determine the enthalpy change (AH₂) for the final reaction below. a. CO(g) + ½ O₂(g) → CO₂(g) AH, -67.6 kcal b. N₂(g) + O₂(g) →→→ 2NO(g) AH₂ = 43.2 kcal c. CO(g) + NO(g) →→→ CO₂(g) + ¹½ N₂(g) AH₂ = ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY