
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
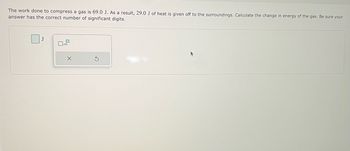
Transcribed Image Text:The work done to compress a gas is 69.0 J. As a result, 29.0 J of heat is given off to the surroundings. Calculate the change in energy of the gas. Be sure your
answer has the correct number of significant digits.
J
☐x10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The work done to compress a gas is 70 J. As a result, 25 J of heat is given off to the surroundings. Calculate the change in energy of the gas.arrow_forwardAn electrical heater is used to supply 235.0 J of energy to a 15.0 g sample of water, originally at 295.0 K. Determine the final temperature in degrees Kelvin. (the specific heat capacity for water is 75.291 Jmol-1K-1)arrow_forwardIt takes 2.16 J of heat to raise the temperature of Object A by 1 °C, and 4.64 J to raise the temperature of Object B by 1 °C. Suppose A and B are brought into contact. A is initially hotter. A is seen to cool down by 6.9 °C. How would you calculate the rise in temperature of B? Set the math up. But don't do any of it. Just leave your answer as a math expression. Also, be sure your answer includes all the correct unit symbols. change in temperature of B = 0 Continue OO X 9 0.0 3 wevee muuiầw Hill LLC. All Rights Reserved. Terms of Use Submit Assignment Accessibility Privacy Center ? doarrow_forward
- Calculate the energy required to heat 775.0 mg of iron from 0.5 °C to 11.2 °C. Assume the specific heat capacity of iron under these conditions is 1 0.449 J.g K Round your answer to 3 significant digits. 0 ロ・ロ X μarrow_forwardbe sure your answer is correct and have the correct number of significant digitsarrow_forwardA chemist carefully measures the amount of heat needed to raise the temperature of a 1.54 kg sample of C5H₁2S from 5.8 °C to 17.4 °C. The experiment shows that 3.51 × 104 J of heat are needed. What can the chemist report for the molar heat capacity of C5H₁2S? Be sure your answer has the correct number of significant digits. 12 - 1 – 1 K J. mol x10 X Ś 6 0 Uarrow_forward
- Calculate the energy required to heat 403.0 mg of water from 36.8 °C to 57.0 °C. Assume the specific heat capacity of water under these conditions is 4.18 J-g .K . Be sure your answer has the correct number of significant digits. 0 0x10 ロ・ロ X μ 00 4 Sarrow_forwardCalculate the energy required to heat 718.0 mg of water from 46.0 °C to 56.7 °C. Assume the specific heat capacity of water under these conditions is -1 -1 4.18 J∙g¯¹·K-¹ Be sure your answer has the correct number of significant digits. 0 x10 X μ 010 ? 00. 18 Ararrow_forwardCalculate the energy required to heat 1.10 kg of water from 49.1 °C to 62.6 °C. Assume the specific heat capacity of water under these conditions is 4.18 J-g ¹K¹. Round your answer to 3 significant digits. 1 H X 10 S 5 ob Ararrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY