
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
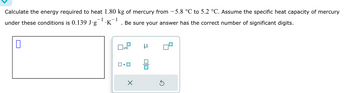
Transcribed Image Text:Calculate the energy required to heat 1.80 kg of mercury from −5.8 °℃ to 5.2 °C. Assume the specific heat capacity of mercury
1
1
under these conditions is 0.139 J∙g¯¹·K¯¹. Be sure your answer has the correct number of significant digits.
0
x10
X
μ
Ś
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- It takes 2.16 J of heat to raise the temperature of Object A by 1 °C, and 4.64 J to raise the temperature of Object B by 1 °C. Suppose A and B are brought into contact. A is initially hotter. A is seen to cool down by 6.9 °C. How would you calculate the rise in temperature of B? Set the math up. But don't do any of it. Just leave your answer as a math expression. Also, be sure your answer includes all the correct unit symbols. change in temperature of B = 0 Continue OO X 9 0.0 3 wevee muuiầw Hill LLC. All Rights Reserved. Terms of Use Submit Assignment Accessibility Privacy Center ? doarrow_forwardA sheet of gold weighing 9.0 g and at a temperature of 13.8 °C is placed flat on a sheet of iron weighing 20.2 g and at a temperature of 57.8 °C. What is the final temperature of the combined metals? Assume that no heat is lost to the surroundings. Be sure your answer has the correct number of significant digits. GC ☐ x10 ☑ ملام 18 Ar 日。 ☑arrow_forwardCalculate the energy required to heat 775.0 mg of iron from 0.5 °C to 11.2 °C. Assume the specific heat capacity of iron under these conditions is 1 0.449 J.g K Round your answer to 3 significant digits. 0 ロ・ロ X μarrow_forward
- be sure your answer is correct and have the correct number of significant digitsarrow_forwardCalculate the energy required to heat 403.0 mg of water from 36.8 °C to 57.0 °C. Assume the specific heat capacity of water under these conditions is 4.18 J-g .K . Be sure your answer has the correct number of significant digits. 0 0x10 ロ・ロ X μ 00 4 Sarrow_forwardA 5.40-g sample of octane is burned in a bomb calorimeter containing 6.17 x 102 g H,O. Given that 1.90 x 10 cal of energy is released if the water temperature increases 3.08°C. Calculate the energy released in J. Enter your answer in scientific notation. Be sure to answer all parts. × 10 (select)arrow_forward
- Calculate the energy required to heat 1.30 kg of iron from 2.3 °C to 11.7 °C. Assume the specific heat capacity of iron under these conditions is 0.449 J∙g¯¹·K¯¹. Be sure your answer has the correct number of significant digits. 1 1 0 x10 ロ・ロ X н 00 JU:::| 00arrow_forwardEnter your answer in the provided box. High-purity benzoic acid (CHgCOOH; AH for combustion =-3227 kJ/mol) is used as a standard for calibrating bomb calorimeters. A 1.221-g sample burns in a calorimeter (heat capacity = 1365 JPC) that contains exactly 1.700 kg of water. What temperature change is observed?arrow_forwardCombustion of natural gas (primarily methane) occurs in most household heaters. The heat given off in this reaction is used to raise the temperature of the air in the house. Part A Assuming that all the energy given off in the reaction goes to heating up only the air in the house, determine the mass of methane required to heat the air in a house by 10.0 ∘C. Assume each of the following: house dimensions are 25.0 m×30.0 m×3.2 m; specific heat capacity of air is 30 J/K⋅mol; 1.00 molof air occupies 22.4Lfor all temperatures concerned. Express your answer using two significant figures. mm = garrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY