
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Calculate the energy, in joules, to heat a cube of gold with a volume in of 12.0cm^3 from 15 Celsius to 29 Celsius. ( Assume that the density of gold is 19.3g/cm^3 , the specific heat for gold is 0.129J/ g Celsius.)
Expert Solution
arrow_forward
Step 1
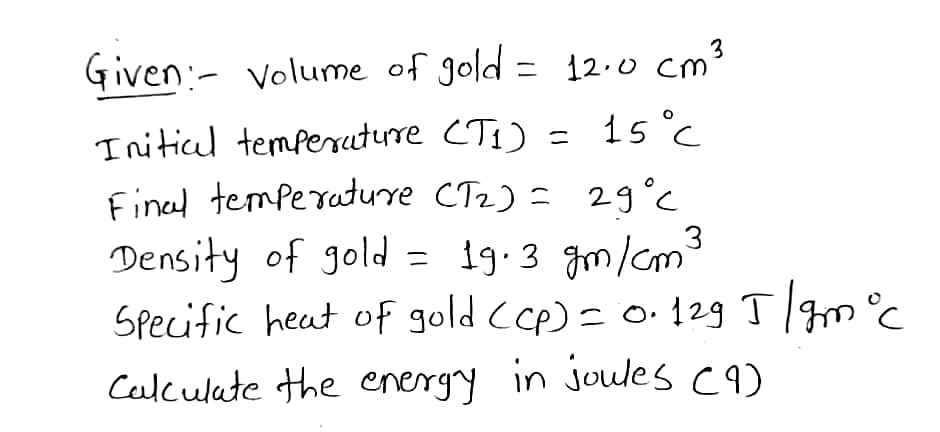
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Similar questions
- Calculate the heat (q) for 195 grams of pure water heated from a temperature of 25.0 °C to 77.5 °C.The specific heat of water is 4.184 J/(g°C).arrow_forwardIf 171.4 grams of water must be heated from 8.00°C to 85.0 °C to make a cup of tea, how much heat must be added to the water? The specific heat capacity of water is 4.184 J/g°Carrow_forwardA 15.0-mL sample of benzene at 21.0 °C was cooled to its melting point, 5.5 °C, and then frozen. How much energy was given off as heat in this process? (The density of benzene is 0.80 g/mL, its specific heat capacity is 1.74 J/g · K, and its heat of fusion is 127 J/g.)arrow_forward
- Explain what the equation Q = m•C•ΔT means and how it relates to energy and calorimetry.arrow_forwardA 55.0g sample of metal z requires 675j of energy to heat it from 25.0 to 118.0 Celsius? Calculate the specific heat of metal z.arrow_forwardIt took 4.15x 10^3 J energy to raise the temperature of a piece of copper metal from 31 degrees Celcius to 84 degrees Celcius. What is the mass of the piece of metal? The specific heat of copper is .0908 cal/g degrees Celcius.arrow_forward
- A student measures the following data regarding the heat of fusion of ice: 24.8 g ice at 0.0°C is placed into the calorimeter which contains 100.0 g water at 21.5°C. The final temperature comes to 2.0°C. The calorimeter has a heat capacity of 15.4 J/°C.arrow_forwardIn the laboratory a student finds that it takes 14.8 Joules to increase the temperature of 10.6 grams of liquid mercury from 24.4 to 35.4 degrees Celsius.The specific heat of mercury calculated from her data isarrow_forwardA 46.0-g sample of copper at 99.2 °C is dropped into a beaker containing 159 g of water at 18.9 °C. What is the final temperature when thermal equilibrium is reached? (The specific heat capacities of liquid water and copper are 4.184 J/g. K and 0.385 J/g • K, respectively.) Final temperature = сarrow_forward
- In the laboratory a student uses a "coffee cup" calorimeter to determine the specific heat of a metal. She heats 18.2 grams of iron to 98.93°C and then drops it into a cup containing 83.9 grams of water at 23.65°C. She measures the final temperature to be 25.28°C. J/g°C. Assuming that all of the heat is transferred to the water, she calculates the specific heat of iron to be ningarrow_forwardA 44.5-g sample of copper at 99.4 °C is dropped into a beaker containing 150. g of water at 18.0 °C. What is the final temperature when thermal equilibrium is reached? (The specific heat capacities of liquid water and copper are 4.184 J/g K and 0.385 J/g · K, respectively.) Final temperature = °Carrow_forwardA serving of Chocobits cereal is 122 g.A 1.00-g sample of the cereal is burned in a bomb calorimeter that has a heat capacity, C, of 8.50 kJ K-1, causing the temperature to increase from 25.0°C to 25.8°C. Calculate the energy of Chocobits in Calories per serving to three significant figures. 1 Cal = 4.184 kJarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY