
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN: 9781305580343
Author: Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
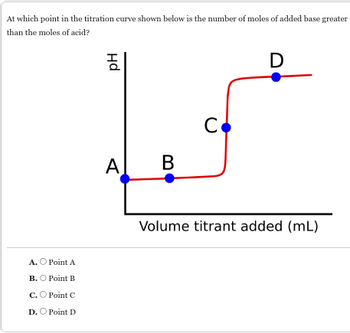
Transcribed Image Text:At which point in the titration curve shown below is the number of moles of added base greater
than the moles of acid?
Hd
A. Point A
B. Point B
C. Point C
D. Point D
A
B
C
D
Volume titrant added (mL)
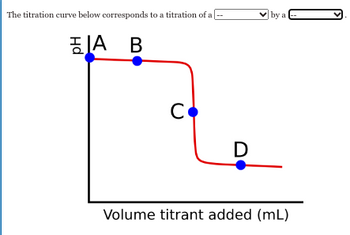
Transcribed Image Text:The titration curve below corresponds to a titration of a
A B
C
D
by a
Volume titrant added (mL)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Consider the nanoscale-level representations for Question 110 of the titration of the aqueous weak acid HX with aqueous NaOH, the titrant. Water molecules and Na+ ions are omitted for clarity. Which diagram corresponds to the situation: After a very small volume of titrant has been added to the initial HX solution? When enough titrant has been added to take the solution just past the equivalence point? Halfway to the equivalence point? At the equivalence point? Nanoscale representations for Question 110.arrow_forwardConsider the nanoscale-level representations for Question 111 of the titration of the aqueous strong acid HA with aqueous NaOH, the titrant. Water molecules and Na+ ions are omitted for clarity. Which diagram corresponds to the situation: (a) After a very small volume of titrant has been added to the initial HA solution? (b) Halfway to the equivalence point? (c) When enough titrant has been added to take the solution just past the equivalence point? (d) At the equivalence point? Nanoscale representations for Question 111.arrow_forwardConsider the titration of HF (K a=6.7104) with NaOH. What is the pH when a third of the acid has been neutralized?arrow_forward
- Follow the directions of Question 64. Consider two beakers: Beaker A has a weak acid(K a=1105). Beaker B has HCI. The volume and molarity of each acid in the beakers are the same. Both acids are to be titrated with a 0.1 M solution of NaOH. (a) Before titration starts (at zero time), the pH of the solution in Beaker A is the pH of the solution in Beaker B. (b) At half-neutralization (halfway to the equivalence point), the pH of the solution in Beaker A the pH of the solution in Beaker B. (c) When each solution has reached its equivalence point, the pH of the solution in Beaker A the pH of the solution in Beaker B. (d) At the equivalence point, the volume of NaOH used to titrate HCI in Beaker B the volume of NaOH used to titrate the weak acid in Beaker A.arrow_forwardAnother way to treat data from a pH titration is to graph the absolute value of the change in pH per change in milliliters added versus milliliters added (pH/mL versus mL added). Make this graph using your results from Exercise 61. What advantage might this method have over the traditional method for treating titration data?arrow_forwardExplain why even though an aqueous acetic acid solution contains acetic acid and acetate ions, it cannot be a buffer.arrow_forward
- The titration of 0.100 M acetic acid with 0.100 M NaOH is described in the text. What is the pH of the solution when 35.0 mL of the base has been added to 100.0 mL of 0.100 M acetic acid?arrow_forwardSketch the titration curve for a weak acid titrated by a strong base. When performing calculations concerning weak acidstrong base titrations, the general two-slep procedure is to solve a stoichiometry problem first, then to solve an equilibrium problem to determine the pH. What reaction takes place in the stoichiometry part of the problem? What is assumed about this reaction? At the various points in your titration curve, list the major species present after the strong base (NaOH, for example) reacts to completion with the weak acid, HA. What equilibrium problem would you solve at the various points in your titration curve to calculate the pH? Why is pH 7.0 at the equivalence point of a weak acid-strong base titration? Does the pH at the halfway point to equivalence have to be less than 7.0? What does the pH at the halfway point equal? Compare and contrast the titration curves for a strong acidstrong base titration and a weak acidstrong base titration.arrow_forwardWhat is meant by the capacity of a buffer? Describe a buffer with low capacity and the same buffer with greater capacity.arrow_forward
- Identify each pair that could form a buffer. (a) NaOH and NaCl (b) NaOH and NH3 (c) Na3PO4 and Na2HPO4arrow_forwardThe three flasks shown below depict the titration of an aqueous NaOH solution with HCl at different points. One represents the titration prior to the equivalence point, another represents the titration at the equivalence point, and the other represents the titration past the equivalence point. (Sodium ions and solvent water molecules have been omitted for clarity.) a Write the balanced chemical equation for the titration. b Label each of the beakers shown to indicate which point in the titration they represent. c For each solution, indicate whether you expect it to be acidic, basic, or neutral.arrow_forwardInstead of the titration of a strong acid by a strong base considered in Question 5, consider the titration of a strong base by a strong acid. Compare and contrast a strong acidstrong base titration with a strong basestrong acid titration.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning