
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Complete each reaction giving the product or the reactants, whichever applies.
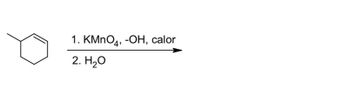
Transcribed Image Text:1. KMnO4, -OH, calor
2. H₂O
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Which is the axidizing agent in the combustion of methanol? O CO O CHOHarrow_forwardThe transformation above can be performed with some combination of the reagants listed below. Give the necessary reagants in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. 8-6 The transformation above can be performed with some combination of the reagents listed below. Give the necessary reagents in the correct order, as a string of letters (without spaces or punctuation, such as "EBF"). If there is more than one correct solution, provide just one answer. A. conc. H₂SO4, heat D. 1) RCO3H; 2) H3O+ B. NaOMe E. HBr C. HBr, ROOR F. t-BUOKarrow_forwardYou run the following reaction with the specific amount of reagents shown in the chemical equation. Based on this information, what would you expect the theoretical yield to be for the major organic product? Br Br2 Br CH2CI2 ČI ČI 480 mg Yield? limiting reagent 0.48 g 480 g O 0.98 g O 1.2 garrow_forward
- What are the products from the complete combustion of propane? C2H2 and H2O CO and H2O CO and H2 CO2 and H2O CO2 and H2arrow_forwardWhy?arrow_forwardEthanol, C₂H60, is most often blended with gasoline - usually as a 10 percent mix - to create a fuel called gasohol. Ethanol is a renewable resource and ethanol-blended fuels, like gasohol, appear to burn more efficiently in combustion engines. The combustion of one mole of ethanol releases 326.7 kcal of energy. The combustion of one mole of 2-methylheptane, C8H18, releases 1.306 × 10³ kcal of energy. How much energy is released during the complete combustion of 405 grams of 2- methylheptane? kcal Assuming the same efficiency, would 405 grams of ethanol provide more, less, or the same amount of energy as 405 grams of 2-methylheptane? more less the same amount Retry Entire Group 9 more group attempts remainingarrow_forward
- Complete and identify the type of reactions. Name all the products and reactants.arrow_forwardThis molecule can be the product of which two organic compounds?arrow_forwardHow many moles of CO2 will be formed in the complete combustion of 4.35 moles of C6H6? Use the following chemical equation to answer this question: 2C6H6+15O2⟶12CO2+6H2O Group of answer choices 1.45 moles 0.725 moles 26.1 moles 13.1 molesarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY