
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
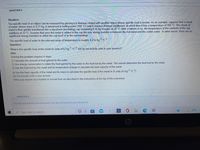
Transcribed Image Text:QUESTION 4
Situation:
The specific heat of an object can be measured by placing it in thermal contact with another object whose specific heat is known. As an example, suppose that a chunk
of metal, whose mass is 0.21 kg, is immersed in boiling water (100 °C) until it reaches thermal equilibrium, at which time it has a temperature of 100 °C. The chunk of
metal is then quickly transferred into a styrofoam (insulating) cup containing 0.18 kg of water at 20 °C. After a minute or so, the temperature of the contents of the cup
stabilizes at 33 °C. Assume that once the metal is added to the cup the only energy transfer is between the hot metal and the colder water. In other words, there are no
significant energy transfers to either the cup itself or to the surroundings.
The specific heat of water in the relevant range of temperature is roughly 4.2 kJ kg 1 °c-1,
Question:
What is the specific heat of the metal (in units of kJ kg1 °C-1, but do not include units in your answer)?
Hint:
Solving this problem requires 4 steps:
1) Calculate the amount of heat gained by the water.
2) Use energy conservation to relate the heat gained by the water to the heat lost by the metal. This should determine the heat lost by the metal.
3) Use the heat lost by the metal and its temperature change to calculate the heat capacity of the metal.
4) Use the heat capacity of the metal and its mass to calculate the specific heat of the metal in SI units (kJ kg1 °C1).
Do not include units in your answer.
Write your answer as a number in normal form as described in the instructions at the top of this worksheet.
QUESTION 5
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
22°F
P Type here to search
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- The thermal conductivities of human tissues vary greatly. Fat and skin have conductivities of about 0.20 W/m · K and 0.020 W/m · K respectively, while other tissues inside the body have conductivities of about 0.50 W/m · K. Assume that between the core region of the body and the skin surface lies a skin layer of 1.0 mm, fat layer of 0.50 cm, and 3.2 cm of other tissues. (a) Find the R-factor for each of these layers, and the equivalent R-factor for all layers taken together, retaining two digits. Rskin m2 · K/W Rfat m2 · K/W Rtissue m2 · K/W R m2 · K/W (b) Find the rate of energy loss when the core temperature is 37°C and the exterior temperature is 0°C. Assume that both a protective layer of clothing and an insulating layer of unmoving air are absent, and a body area of 2.0 m2. Warrow_forwardA block of metal of mass 0.360 kg is heated to 144.0°C and dropped in a copper calorimeter of mass 0.250 kg that contains 0.170 kg of water at 30°C. The calorimeter and its contents are insulated from the environment and have a final temperature of 44.0°C upon reaching thermal equilibrium. Find the specific heat of the metal. Assume the specific heat of water is 4.190 x 103 J/(kg · K) and the specific heat of copper is 386 J/(kg · K). J/(kg · K)arrow_forwardA block of metal of mass 0.280 kg is heated to 148.0°C and dropped in a copper calorimeter of mass 0.250 kg that contains 0.190 kg of water at 30°C. The calorimeter and its contents are insulated from the environment and have a final temperature of 44.0°C upon reaching thermal equilibrium. Find the specific heat of the metal. Assume the specific heat of water is 4.190 x 103 J/(kg · K) and the specific heat of copper is 386 J/(kg · K). 3/(kg K)arrow_forward
- A 390-g metal container, insulated on the outside, holds 170.0 g of water in thermal equilibrium at 21.0°C. A 18.0-g ice cube, at -15.0°C, is dropped into the water, and when thermal equilibrium is reached the temperature is 12.0°C. Assume there is no heat exchange with the surroundings. The specific heat capacity of water is 4190 J/kg ∙ K, the specific heat capacity of ice is 2090 J/kg ∙ K and the heat of fusion is 3.34 × 105 J/kg. What is the specific heat capacity of the metal of the container?arrow_forwardAn insulated container is partly filled with oil. The lid of the container is removed, 0.150 kg of water heated to 84.0°C is poured in, and the lid is replaced. As the water and the oil reach equilibrium, the volume of the oil increases by 1.00x105 m³. The density of the oil is 919 kg/m³, its specific heat capacity is 1980 J/(kg-C°), and its coefficient of volume expansion is 726x10-6 (Cº)-¹. What is the temperature when the oil and the water reach equilibrium? Number Unitsarrow_forwardA 0.250-kg aluminum bowl holding 0.800 kg of soup at 26.6°C is placed in a freezer. What is the final temperature if 420 kJ of energy is transferred from the bowl and soup? Assume the soup has the same thermal properties as that of water, the specific heat of the liquid soup is 1.00 kcal/(kg · °C), frozen soup is 0.500 kcal/(kg · °C), and the latent heat of fusion is 79.8 kcal/kg. The specific heat of aluminum is 0.215 kcal/(kg · °C).arrow_forward
- A copper block with a mass of 400 grams is cooled to 77 K by being immersed in liquid nitrogen. The block is then placed in a Styrofoam cup containing some water that is initially at +50.0°C. Assume no heat is transferred to the cup or the surroundings. The specific heat of liquid water is 4186 J/(kg °C), of solid water is 2060 J/(kg °C), and of copper is 385 J/(kg °C). The latent heat of fusion of water is 3.35 x 105 J/kg. What is the mass of water in the cup, if the final temperature is -25.0°C?arrow_forwardA block of metal of mass 0.200 kg is heated to 152.0°C and dropped in a copper calorimeter of mass 0.250 kg that contains 0.150 kg of water at 30°C. The calorimeter and its contents are insulated from the environment and have a final temperature of 46.0°C upon reaching thermal equilibrium. Find the specific heat of the metal. Assume the specific heat of water is 4.190 × 103 J/(kg · K) and the specific heat of copper is 386 J/(kg · K). J/(kg · K)arrow_forwardIce at 0 °C is placed in a Styrofoam cup containing 0.62 kg of lemonade at 32 °C. The specific heat capacity of lemonade is virtually the same as that of water; that is, c = 4180 J/(kg C°). After the ice and lemonade reach an equilibrium temperature, some ice still remains. The latent heat of fusion for water is Lf = 3.35 x 105 J/ kg. Assume that the mass of the cup is so small that it absorbs a negligible amount of heat, and ignore any heat lost to the surroundings. Determine the mass of ice that has melted, in grams.arrow_forward
- Water is placed into an iron container along with an ice cube. They are thermally isolated.The iron container has a mass of 790. g and is initially at 72.0 degrees Celsius. The water has a mass of 220. g and is initially at 17.0 degrees Celsius. The ice cube has a mass of 80.0 g and is initially at -15.0 degrees Celsius.What is the heat required to raise the temperature of the ice to 0 degrees Celsius?arrow_forwardA 0.100 kg piece of ice at initial temperature −5.00 ◦C is placed in a perfectly insulated container with 1.00 kg (1 L) of water at initial temperature 20.0 ◦C. The container absorbs or releases no heat. All of the ice melts as the system reaches an equilibrium temperature Tf . How much heat must the ice exchange with the rest of the system to raise its temperature to the melting point, 0.00 ◦C? Would this heat exchanged be positive, zero, or negative? Once the ice reaches its melting point, how much heat must the ice exchange with the rest of the system to melt? Would this heat exchanged be positive, zero, or negative? Once the ice melts, it is liquid water at 0.00 ◦C. Write an expression for the heat exchanged by the newly-melted ice QI to reach the equilibrium temperature Tf . Would you expect QI to be positive, zero, or negative? Write an expression for the heat exchanged by the original water QW to reach the equilibrium temperature Tf from its initial temperature. Assuming no…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON