
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
please include the values for i,j,k to complete the table. thank you!
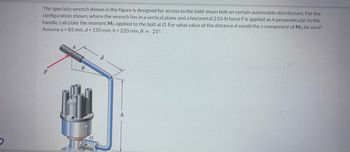
Transcribed Image Text:The specialty wrench shown in the figure is designed for access to the hold-down bolt on certain automobile distributors. For the
configuration shown, where the wrench lies in a vertical plane and a horizontal 210-N force F is applied at A perpendicular to the
handle, calculate the moment Mo applied to the bolt at O. For what value of the distance d would the z-component of Mo be zero?
Assume a = 85 mm, d = 150 mm, h = 220 mm, 0 = 21°
F
8
h
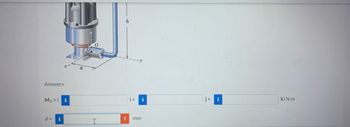
Transcribed Image Text:Answers
Moi
d = i
I
h
i+ i
! mm
j+
k) N-m
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Please show all your work neatly including finding the correct units. for : A% = 40+51, so A =91% for : P_LA=B hPa. B= 0+1012, so B = 1012 for : P_denver=C hPa. C=3+838, so C =841arrow_forwardBuild the following part. Report the mass in pounds. Assume all dimensions are in inches. The material is Vanadium. (Located in the "Other Metals" folder in the material properties.) A = 1.72 B = 1.4 2.40 1.20 T .75 TYP 1.20 TYP .70 -1.00 TYP A A A+3.25 R.35 TYP -R.65 TYP CBORE FOR 5/16 HEX HEAD BOLT Ø.332 THRU ALL LØ813 V 235 .10- SECTION A-A 23. B B+1arrow_forward2. Complete the table. This problem involves the power required to raise a mass a given distance in a given amount of time. (Round your answers for mass and energy to the nearest whole number.) (Round your answers for power to two decimal places.) 3 Click the icon to view the table to complete. 4 Click the icon to view the conversion table. 5 Click the icon to view the table of common derived units in the Sl system. 3: More Info 4: More Info 2 11 Q W (a) (b) (a) (b) Mass [bm] 220 Time 1 d = 24 h 1 h = 60 min Mass [Ibm 1 min = 60 s 3 1 yr = 365 d E 220 $ 4 Distance [ft] 1 kg = 1 slug = 14.5939 kg 1 slug = 32.2 lbm 15 Distance [ft] 15 35 Mass 2.205 lbm I R 35 % 5 I T Energy [J] Length 1 m = 3.28 ft 1 km = 0.621 mi 1 in = 2.54 cm 1 mi = 5,280 ft Energy [J] ^ 6 MacBook Pro Y & 7 Time [min] Energy 1 J = 0.239 cal = 9.48 x 10-4 BTU = 0.7376 ft lbf 1 kWh 3,600,000 J H 0.5 Time [min] 0.5 2 2 8 1 ( 20 Power [hp] 0.134 Power [hp] 0.134 Power 1 W 3.412 BTU/h = 0.00134 hp = 14.34 cal/min = 0.7376…arrow_forward
- An electric hot water heater consumes 3.1 kilowatts of electricity and converts it to heat. How long will it take the water heater to heat a 67 gallon tank of water from 10 degrees Celsius to 50 degrees Celsius? (1 kilogram of water is 0.37 gallons, 1 Calorie = 4200 J). It may be helpful to refer back to the weekly handout for guidance on this problem. Your final answer should be in minutes (rounded to the nearest 10 minutes).arrow_forwardPlease look at the images for details about the question and the needed diagram. Make sure to include 2 places after the decimal point.arrow_forwardConvert 0.226 ug into mg. Report answer in scientific notation. The decimal part is -6 expressed with the letter "e" in the answer For example 5.104 X 10° would be written 5.104e-6. This seems to be the only way Canvas understands scientific notation.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY