
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
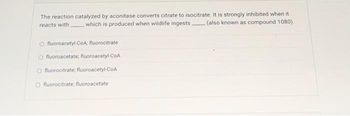
Transcribed Image Text:The reaction catalyzed by aconitase converts citrate to isocitrate. It is strongly inhibited when it
reacts with which is produced when wildlife ingests (also known as compound 1080),
fluoroacetyl-CoA; fluorocitrate
O fluoroacetate, fluoroacetyl-CoA
Ofluorocitrate, fluoroacetyl-CoA
Ofluorocitrate, fluoroacetate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- Pyruvate + NAD+ + CoA → Acetyl COA NAIDHI + H + CO₂ This is the overall reaction for the pathway of Glycolysis Oxidative decarboxylation of pyruvate Fatty acid oxidation Ellectron transport Oxidative phosphorylation TCA or citric acid cyclearrow_forwardAcetyl CoA 00-COA COA Six -carbon molecule formed from acetyl CoA + oxaloacetate Four-carbon acceptor molecule (regenerated in each cycle) O00000 Citrate One carbon lost as 5 co2 O000 Oxaloacetate NAD+ NADH NADH + CO2 NAD* Secand carbon NAD+ lost as COz NADH + COD Reduced electron carrier similar to NADH Z7 GDP, O FADH2 Energy-carrying n molecule equivalent FAD GTP to ATParrow_forwardAlpha-ketoglutarate dehydrogenase exhibits feedback inhibition byarrow_forward
- Which of the following glycolytic enzymes catalyzes the conversion of fructose-1,6-bisphosphate into the two product molecules dihydroxyacetone phosphate, and glyceraldehyde-3-phosphate? phosphoglycerate kinase pyruvate kinase malate dehydrogenase aldolase phosphofructokinasearrow_forwardWhich of the following statements is true for the shown reactions? Deficiency of oxaloacetate stimulates the formation of X and y Insulin signaling stimulates the formation of X and Y Both A and B Neither A nor Barrow_forwardWhich citric acid cycle enzyme utilizes a similar reaction mechanism to pyruvate dehydrogenase? O Malate dehydrogenase Isocitrate dehydrogenase O Alpha-ketoglutarate dehydrogenase Succinate dehydrogenasearrow_forward
- The following reaction, the last reaction in beta-oxidation, is an example of a: Enzyme Enzyme-H S-CoA S-COA H2C H3C CoA s–CoA C H2 CH2 R S-COA S-COA S-COA O A. Nucleophilic acyl substitution O B. Electrophilic addition OC. Nucleophilic addition D. Aldol condensation O E. Hydrohalogenationarrow_forwardUnder anaerobic conditions, following the radiolabel at carbon-2 of pyruvate, in which molecule does the carbon label ultimately end up so that it can be taken up by the muscle cell and used for metabolism? Lactate C-2 Glucose C-2 and C-5 Acetyl- CoA C-2 Glucose C-1 and C-6 CO2 Pyruvate C-2arrow_forward[References) Which of the following statements explains why acetyl-CoA Iis considered the central molecule of metabolism? (Select all that apply.) O The addition of acetyl-CoA to compounds stabilises them. Acetyl-CoA contains a thioester bond that makes it one of the high energy compounds in metabolic pathways. O Acetyl-CoA is an inhibitor that plays a central role in regulating metabolic pathways. O Acetyl-CoA feeds directly into the citric acid cycle. U None of the above.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON