Question
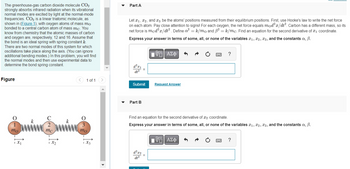
Transcribed Image Text:The greenhouse-gas carbon dioxide molecule CO2
strongly absorbs infrared radiation when its vibrational
normal modes are excited by light at the normal-mode
frequencies. CO₂ is a linear triatomic molecule, as
shown in (Figure 1), with oxygen atoms of mass mo
bonded to a central carbon atom of mass mc. You
know from chemistry that the atomic masses of carbon
and oxygen are, respectively, 12 and 16. Assume that
the bond is an ideal spring with spring constant k.
There are two normal modes of this system for which
oscillations take place along the axis. (You can ignore
additional bending modes.) In this problem, you will find
the normal modes and then use experimental data to
determine the bond spring constant.
Figure
O
1
mo
1x₁
2
mc
1 of 1
3
mo
1Xz
Part A
Let x₁, x2, and 3 be the atoms' positions measured from their equilibrium positions. First, use Hooke's law to write the net force
on each atom. Pay close attention to signs! For each oxygen, the net force equals mod²x/dt². Carbon has a different mass, so its
net force is mcd²x/dt². Define a² = k/mo and 3² = k/mc. Find an equation for the second derivative of ₁ coordinate.
Express your answer in terms of some, all, or none of the variables ₁, 2, 3, and the constants a, ß.
17 ΑΣΦ
d²x₁
dt²
Submit
Part B
d²x2
dt²
Request Answer
Find an equation for the second derivative of 2 coordinate.
Express your answer in terms of some, all, or none of the variables ₁, 2, 3, and the constants a, B.
=
?
——| ΑΣΦ
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- H1.arrow_forwardIn vibrational spectroscopy, the fundamental band refers to a transition from the n = 0 state to n = 1, and the first overtone would be from the n = 0 state to n = 2. For carbon monoxide (CO), the fundamental occurs at 2143.4 cm-¹ and the first overtone at 4259.6 cm-¹. (a) For an anharmonic oscillator, the energy levels can be written (in wavenumbers) as 2 En ) ²³ = ve (n + ¹) - xeve (n +. + Find the values of ve and xeve from the fundamental and overtone transitions in CO. Report your results in units of cm-¹. (b) Use the values found for ve and xeve in part (a) to estimate the number of bound energy levels in CO. (Hint: For an anharmonic oscillator this number is determined by the point at which the spacing between consecutive levels goes to zero.) (c) If CO could be modeled as a Morse oscillator, and the values of ve and xeve provide energy levels consistent with the Morse potential what would be the value of the parameter D in units of cm-¹? How does this compare with the observed…arrow_forwardHow do I solve for Problem 12.1? This problem is in a chapter called "Molecules," and it is part of a portion called Systems with two or more Atoms.arrow_forward
- Suppose the distance between the two atoms is equal to the equilibrium distance found in part A. What minimum energy must be added to the molecule to dissociate it-that is, to separate the two atoms to an infinite distance apart? This is called the dissociation energy of the molecule. For the molecule CO, the equilibrium distance between the carbon and oxygen atoms is 1.13×10−10m and the dissociation energy is 1.54×10−18J per molecule. Find the value of the constant a. Find the value of the constant b.arrow_forwardP9E.11 (a) For a linear conjugated polyene with each of N carbon atoms contributing an electron in a 2p orbital, the energies E, of the resulting A molecular orbitals are given by: E, =a+2B cos- N+1 k=1, 2,.,N Use this expression to make a reasonable empirical estimate of the resonance integral B for the homologous series consisting of ethene, butadiene, hexatriene, and octatetraene given that t-n ultraviolet absorptions from the HOMO to the LUMO occur at 61 500, 46 080, 39 750, and 32 900 cm", respectively. (b) Calculate the T-electron delocalization energy, Egdo:= E, - n(a+ B), of octatetraene, where E, is the total T-electron binding energy and n is the total number of T-electrons. (c) In the context of this Hückel model, the molecular orbitals are written as linear combinations of the carbon 2p orbitals. The coefficient of the jth atomic orbital in the kth molecular orbital is given by: cN sin j=1,2.N jkn j=1, 2,.,N Evaluate the coefficients of each of the six 2p orbitals in each…arrow_forwardFind the amplitude of the ground-state vibrations of the CO molecule. What percentage of the bond length is this? Assume the molecule vibrates like a harmonic oscillator.arrow_forward
- - Calculate the average number of phonons occupying a vibrational mode with angular fre- quency w = 4.0 x 10¹2 s-1 at T = 300 K. - Calculate the total energy of the mode at this temperature, expressing your answer in meV.arrow_forwardThe ionization energy of a carbon atom is 11.26 eV and its electron affinity is 1.26 eV Estimate the value of the Coulomb integral. α. expressing your answer both in electronvolts and as a molar energy in ki lojoules per mole.arrow_forwardQ.1. The metals Cu and Pb have diamagnetic susceptibilities -9.63×10-6 and -1.58x10-5, respectively. Furthermore, Pb is a superconductor with T=7.2 K and Bo=800 G. Compare the magnetic energies of Cu and Pb in an external field of 500 G at 7.5 K and 0.1 K; assume the long-wire geometry with the field parallel to the wire. What is the principal difference in the free energy of the Pb sample at the two temperatures and in the presence of the field?arrow_forward
arrow_back_ios
arrow_forward_ios