Question
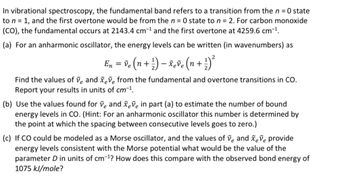
Transcribed Image Text:In vibrational spectroscopy, the fundamental band refers to a transition from the n = 0 state
to n = 1, and the first overtone would be from the n = 0 state to n = 2. For carbon monoxide
(CO), the fundamental occurs at 2143.4 cm-¹ and the first overtone at 4259.6 cm-¹.
(a) For an anharmonic oscillator, the energy levels can be written (in wavenumbers) as
2
En ) ²³
= ve (n + ¹) - xeve (n +.
+
Find the values of ve and xeve from the fundamental and overtone transitions in CO.
Report your results in units of cm-¹.
(b) Use the values found for ve and xeve in part (a) to estimate the number of bound
energy levels in CO. (Hint: For an anharmonic oscillator this number is determined by
the point at which the spacing between consecutive levels goes to zero.)
(c) If CO could be modeled as a Morse oscillator, and the values of ve and xeve provide
energy levels consistent with the Morse potential what would be the value of the
parameter D in units of cm-¹? How does this compare with the observed bond energy of
1075 kJ/mole?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 5 images

Knowledge Booster
Similar questions
- A quantum system is described by a wave function (r) being a superposition of two states with different energies E1 and E2: (x) = c191(r)e iEit/h+ c292(x)e¯iE2t/h. where ci = 2icz and the real functions p1(x) and p2(r) have the following properties: vile)dz = ile)dz = 1, "0 = rp(x)T#(x)l& p1(x)92(x)dx% D0. Calculate: 1. Probabilities of measurement of energies E1 and E2 2. Expectation valuc of cnergy (E)arrow_forward(a)How many sets of quantum numbers are possible for a hydrogen atom for n = 4,? ). (b) Write out a set of possible values for the quantum numbers n, ℓ, mℓ, and ms for each electron if all states are occupied including n=4. (c)Write table of occupancy of quantum numbers: n, ℓ, mℓ, and ms for Arsenic As, including spin orientations.arrow_forwardSuppose that the ground vibrational state of a molecule is modelled by using the particle-in-a-box wavefunction ψ0 = (2/L)1/2 sin(πx/L) for 0 ≤ x ≤ L and 0 elsewhere. Calculate the Franck–Condon factor for a transition to a vibrational state described by the wavefunction ψ′= (2/L)1/2sin{π(x −L/4)/L} for L/4 ≤ x ≤ 5L/4 and 0 elsewhere.arrow_forward
arrow_back_ios
arrow_forward_ios