
Chemistry & Chemical Reactivity
10th Edition
ISBN: 9781337399074
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
please give solution and answer to this question
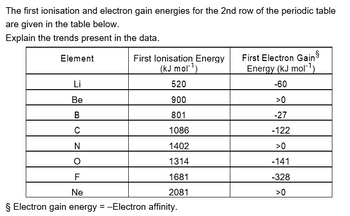
Transcribed Image Text:The first ionisation and electron gain energies for the 2nd row of the periodic table
are given in the table below.
Explain the trends present in the data.
Element
Li
Be
BUZO
C
N
F
First lonisation Energy
(kJ mol-¹)
520
900
801
1086
1402
1314
1681
2081
Ne
§ Electron gain energy = -Electron affinity.
First Electron Gain
Energy (kJ mol-¹)
-60
>0
-27
-122
>0
-141
-328
>0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using the periodic table and without looking at Table 7.3, write electron configurations for the following elements: (a) P (b) Zn (c) Zr (d) In (e) Pb (f) U Use the spdf and noble gas notations. When you have finished, check your answers with Table 7.3.arrow_forwardConsider the following statement "The ionization energy for the potassium atom is negative, because when K loses an electron to become K +, it achieves a noble gas electron configuration." Indicate everything that is correct in this statement. Indicate everything that is incorrect. Correct the incorrect information and explain.arrow_forwardBefore the element scandium was discovered in 1879, it was known as “eka-boron.” Predict the properties of scandium from averages of the corresponding properties of its neighboring elements in the periodic table. Compare your predictions with the observed values in Appendix F.arrow_forward
- Of the five elements Sn, Si, Sb, O, Te, which has the most endothermic reaction? (E represents an atom.) What name is given to the energy for the reaction? E(g)E+(g)+earrow_forwardThe energy needed to remove one electron from a gaseous potassium atom is only about two-thirds as much as that needed to remove one electron from a gaseous calcium atom, yet nearly three times as much energy as that needed to remove one electron from K+ as from Ca+ . What explanation can you give for this contrast? What do you expect to be the relation between the ionization energy of Ca+ and that of neutral K?arrow_forwardFor each of the following pairs of elements, choose the one that correctly completes the following table.arrow_forward
- Cesium is used extensively in photocells and in television cameras because it has the lowest ionization energy of all the stable elements. (a) What is the maximum kinetic energy of a photoelectron ejected from cesium by 520 nm light? Note that if the wavelength of the light used to irradiate the cesium surface becomes longer than 660 nm, no photoelectrons are emitted. (b) Use the rest mass of the electron to calculate the velocity of the photoelectron in (a).arrow_forwardFor each of the following pairs of atoms or ions, state which you expect to have the larger radius. (a) Na or K (b) Cs or Cs+ (c) Rb+ or Kr (d) K or Ca (e) Cl or Ararrow_forwardDoes the information on alkali metals in Table 2-8 of the text confirm the general periodic trends in ionization energy and atomic radius? Explain.arrow_forward
- Group the electronic configurations of neutral elements in sets according to those you would expect to show similar chemical properties. Set A Set B Answer Bank 1s 252p 3s²3p 1s 252p°3s²3p 1s²2s²2p°3s²3p°4s²3d!04p° 1s252p Determine the chemical symbols for the neutral elements corresponding to the electronic configurations. Use proper formatting; letter case matters. 1s²25 2p?: 1s 25 2p°3s²3p°4s²3d!04p°:arrow_forward||| K 91 alaccd sign in - X C esc U 1 O ELECTRONIC STRUCTURE AND CHEMICAL BONDING Writing the electron configuration of an atom using the Periodic... 23 V Write the electron configuration of an atom of the element highlighted in this outline of the Perio 4 f1 5 19 6 7 Explanation Hint: you do not need to know the name or symbol of the highlighted element! ALEKS McGraw-Hill Ed X A ALEKS - Shush. X ps://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IvUrTNdLZh5A8CnG03PBGUXE ? Type here to search f2 2 Check X f3 # 3 Mc Graw HI E f4 GA AUT 4 O R f5 % 4 5 ㄱ 1 0 BE f7 hp 4+ Y 7 ALEKS f8 He Ne Ar Kr Xe Rn X TE © 2022 McGraw Hill LLC. a * 8 DII 9arrow_forwardQ1. This question is about atomic structure. (a) Write the full electron configuration for each of the following species. CH Fe2+ (b) Write an equation, including state symbols, to represent the process that occurs when the third ionisation energy of manganese is measured. (c) State which of the elements magnesium and aluminium has the lower first ionisation energy Explain your answer. (d) A sample of nickel was analysed in a time of flight (TOF) mass spectrometer. The sample was ionised by electron impact ionisation. The spectrum produced showed three peaks with abundances as set out in the table. m/z Abundance /% 58 61.0 60 29.1 61 9.9 Give the symbol, including mass number, of the ion that would reach the detector first in the sample. Calculate the relative atomic mass of the nickel in the sample. Give your answer to one decimal place. Page 2 of 12 Symbol of ion Relative atomic massarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning


Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning