
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
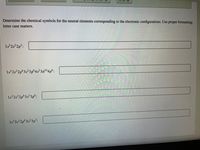
Transcribed Image Text:Determine the chemical symbols for the neutral elements corresponding to the electronic configurations. Use proper formatting;.
letter case matters.
1s2s2p³:
1s 252p°3s²3p°4s²3d104p°:
1s2s 2p°3s²3p°:
1s 25 2p°3s²3p³:
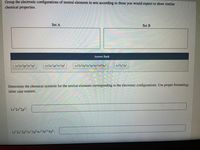
Transcribed Image Text:Group the electronic configurations of neutral elements in sets according to those you would expect to show similar
chemical properties.
Set A
Set B
Answer Bank
1s 252p 3s²3p
1s 252p°3s²3p
1s²2s²2p°3s²3p°4s²3d!04p°
1s252p
Determine the chemical symbols for the neutral elements corresponding to the electronic configurations. Use proper formatting;
letter case matters.
1s²25 2p?:
1s 25 2p°3s²3p°4s²3d!04p°:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Report Sheet: Exp. 15: PERIODIC PROPERTIES NOTE: All chemical equations must be balanced and must include physical states or phases. Name of partner: I. Reactivity with Water A. Lithium, Sodium and Potassium Describe the reaction of each metal with water. Include specific identification of the products formed. Na and H6₂0 Q-1 Q-2 feezing k and H₂0 feezing Li aindl H₂0 feezing Write the balanced chemical equation for each reaction: Q-3 and Q-4 turn smoke andl Pink ignition turn Pink Clear Rank:arrow_forwardHow can I tell the charge of NaCH3COO, NaCl, and HCH3COO? Just in general I am not sure of how to determine the charge of compounds, so if that could be explained?arrow_forwardSelect all the elements whose elemental forms would exist as networks: Hydrogen Helium Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neonarrow_forward
- List the seven diatomic elements. Give their names and symbols.arrow_forwardWhat were the problems with Dobereiner’s triads and Newlands’ law of octaves? Why were these two early attempts to organize elements abandoned in favour of Mendeleev’s periodic table?arrow_forwardI can't fit both sections of the question itto one shot, so please answer both parts. Thank you very mucharrow_forward
- Silicon is the foundation of today's Microelectronics.a) Indicate which other elements from group IV of the periodic table find applications in electronics, pointing out their usefulness.b) Similarly, composite materials using elements from groups III-V are widely used. Name at least 3 of these compounds, exemplifying their respective applications.arrow_forwardDisplay the groups of the periodic table and their charges.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY