
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
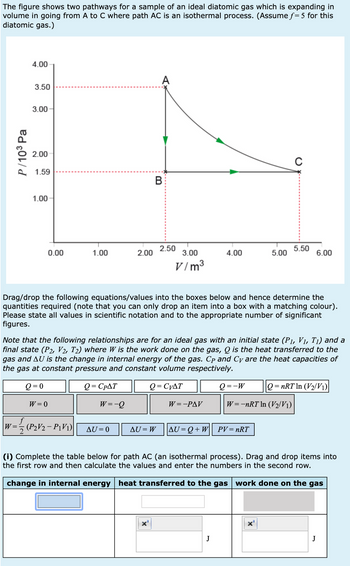
Transcribed Image Text:The figure shows two pathways for a sample of an ideal diatomic gas which is expanding in
volume in going from A to C where path AC is an isothermal process. (Assume f= 5 for this
diatomic gas.)
P/103 Pa
4.00
A
3.50
3.00
2.00
1.59
B
1.00
0
2.50
0.00
1.00
2.00
5.50
3.00
4.00
5.00
6.00
V/m³
Drag/drop the following equations/values into the boxes below and hence determine the
quantities required (note that you can only drop an item into a box with a matching colour).
Please state all values in scientific notation and to the appropriate number of significant
figures.
Note that the following relationships are for an ideal gas with an initial state (P1, V1, T₁) and a
final state (P2, V2, T2) where W is the work done on the gas, Q is the heat transferred to the
gas and AU is the change in internal energy of the gas. Cp and Cy are the heat capacities of
the gas at constant pressure and constant volume respectively.
Q=0
Q= CPAT
Q = CVAT
Q=-w
Q = nRT In (V2/V1)
W=0
W=-Q
W=-PAV
W=-nRT In (V2/V1)
W= (P2V2-P1V1)
AU=0
AU = W AU=Q+W PV = nRT
(i) Complete the table below for path AC (an isothermal process). Drag and drop items into
the first row and then calculate the values and enter the numbers in the second row.
change in internal energy heat transferred to the gas work done on the gas
J
J
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- 10. A container with a movable piston holds 2.00 moles of a monatomic ideal gas at a pressure of 3.0 × 105 N/m2 in a volume of 0.018 m3 . (a) What is the temperature of the gas? (b) The gas undergoes an isothermal expansion to a volume of 0.027 m3 . How much work does the gas do during this expansion? (c) How much heat flows into or out of the gas during this expansion? Does it flow into or out of the gas?arrow_forwardA gas has a constant pressure of 3000Pa. It is isobarically expanded from 0.75m^3 to 1.25m^3. During the process, 100J of thermal energy is added through heat. a) What is the work done on the gas? b) What is the change in internal energy of the gas?arrow_forwardA 1 mol sample of a diatomic ideal gas (γ=1.4) expands slowly and adiabatically from a pressure of 18 atm and a volume of 3 L to a final volume of 18 L. What is the final temprature (in K) of the gas? ( Answer no decimal )arrow_forward
- Question 1. An ideal diatomic gas contracts from 1.25 m³ to 0.500 m³ at a constant pressure of 1.50 x 10°P.. Draw a PV diagram and name this process that occurs at constant pressure. If the initial temperature is 425 K, calculate (a) the work done on the gas, (b) the change in internal energy of the gas, (c) the energy transfer, Q, and, (d) the final temperature.arrow_forwardA sample of n = 2.00 moles of monoatomic ideal gas expands adiabatically, the work done on the gas is W = -5.00 x 103 J. The initial temperature and pressure of the gas are Ti = 600 K and Pi = 4.05 x 105 Pa. Calculate: a) the final temperature of the gas; b) the final pressure of the gas. R = 8.314 J/mol Karrow_forwardA monatomic ideal gas initially fills a V0 = 0.45 m3 container at P0 = 85 kPa. The gas undergoes an isobaric expansion to V1 = 1.4 m3. Next it undergoes an isovolumetric cooling to its initial temperature T0. Finally it undergoes an isothermal compression to its initial pressure and volume. 1 Calculate the work done by the gas, W1, in kilojoules, during the isobaric expansion (first process). 2 Calculate the heat absorbed Q1, in kilojoules, during the isobaric expansion (first process). 3 Write an expression for the change in internal energy, ΔU1 during the isobaric expansion (first process). 4 Calculate the work done by the gas, W2, in kilojoules, during the isovolumetric cooling (second process). 5 Calculate the heat absorbed Q2, in kilojoules, during the isovolumetric cooling (second process). 6 Calculate the change in internal energy by the gas, ΔU2, in kilojoules, during the isovolumetric cooling (second process). 7 Calculate the work done by the gas, W3, in kilojoules,…arrow_forward
- Under constant-volume conditions, 2800 J of heat is added to 1.6 moles of an ideal gas. As a result, the temperature of the gas increases by 140 K. How much heat would be required to cause the same temperature change under constant-pressure conditions? Do not assume anything about whether the gas is monatomic, diatomic, etc.arrow_forwardAs shown below, a nonideal gas goes through the cycle ABCA. During the process AB, 71.5 J of heat was added to the gas. During the process BC, 8.2 J of heat was removed from the gas. Determine WABCA & QCA. WABCA = QCA P(N/m²) 10 2 2 A 4 6 8 B с 10 V (m³)arrow_forwardA sealed ideal gas system contains 2.0 moles of monatomic ideal gas, initially at temperature 300 K and pressure 1.2 atm. The system is allowed to expand isothermally to five times its original volume. How much heat is transferred into the system during this process? 7.09 kJ 11.2 kJ 8.03 kJ Zero 4.97 kJarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON