
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
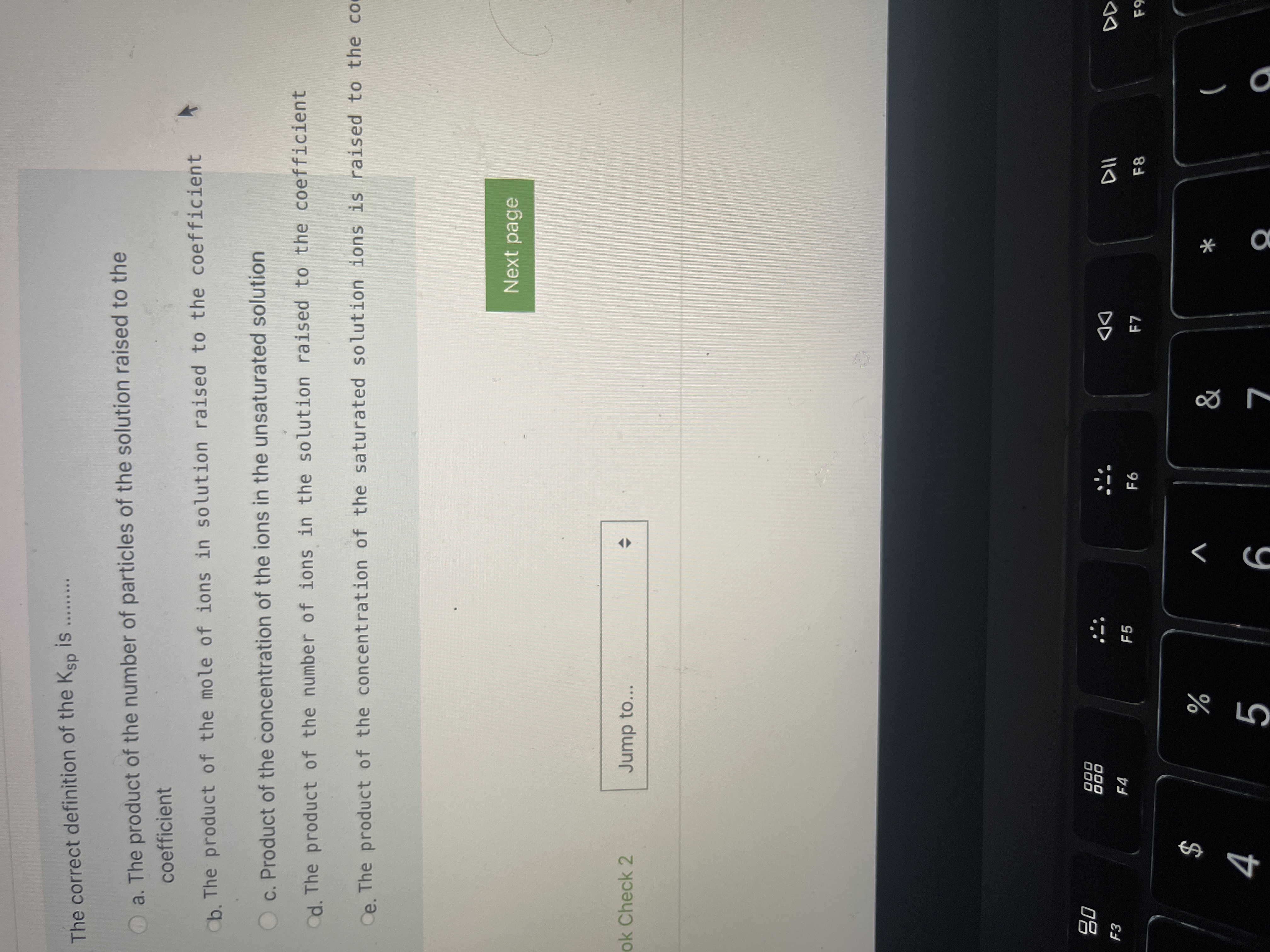
Transcribed Image Text:The correct definition of the Ksp is
.....
a. The product of the number of particles of the solution raised to the
coefficient
Cb. The product of the mole of ions in solution raised to the coefficient
c. Product of the concentration of the ions in the unsaturated solution
d. The product of the number of ions in the solution raised to the coefficient
Ce. The product of the concentration of the saturated solution ions is raised to the co
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The addition of 0.3800 L of 1.150 M KCl to a solution containing Ag+ and Pb2+ ions is just enough to precipitate all of the ions as AgCl and PbCl,. The total mass of the resulting precipitate is 61.40 g. Find the masses of PbCl, and AgCl in the precipitate. mass of PbCl, : g mass of AgCl:arrow_forwardSolid aluminum nitrate is slowly added to 150 mL of a 0.0699 M potassium hydroxide solution. The concentration of aluminum ion required to just initiate precipitation is M.the expert working on my last question, doesnt have to anymore, its been answered thanks.arrow_forward4. A 25.00 mL sample of a weak acid HX is titrated with 0.1250 M NaOH. The equivalence point in the titration occurs when 19.91 mL of the NaOH solution has been added. Neutralization reaction: HX + NaOH → NaX + H₂O Please answer the following questions. A. B. Based on the volume of 0.1250 M NaOH required to reach the equivalence point, calculate the concentration of the weak acid HX in the original 25.00 mL sample. M D. Please provider your answer below. 0² 0, (0) Check answer Determine the concentration of HX in the solution after the 10.00 mL of 0.1250 M NaOH has been added to the original 25.00 mL of weak acid sample in the titration. Please provider your answer below. 号 (0) Check answer Check answer C. Determine the concentration of NaX in the solution after the 10.00 mL of 0.1250 M NaOH has been added to the original 25.00 mL of weak acid sample in the titration. Please provider your answer below. 0₂ ← A ← Check answer $ ← → ← $ Given that Ka = 4.72x10-8 for the weak acid HX,…arrow_forward
- Question 7 pleasearrow_forwardThe maximum amount of lead chloride that will dissolve in a 0.111 M potassium chloride solution is M.arrow_forwardMatch the definition with it's correct word. A mixture composed of a solute dissolved in a solvent. The greater substance that does the dissolving in a solution. A term that describes when a solid solute does not dissolve in a solid solvent. [Choose ] [Choose ] Soluble Solvent Solution Insoluble Miscible Immiscible Solute >arrow_forward
- < Question 39 of 41 The addition of 0.3800 L of 1.150M KCI to a solution containing Ag* and Pb?+ ions is just enough to precipitate all of the ions as AgCl and PbCL,. The total mass of the resulting precipitate is 62.10 g. Find the masses of PbCl, and AgCl in the precipitate. mass of PbCl,: mass of AgCl: A solution contains Al and Co. The addition of 0.3823 L of 1.654 M NAOH results in the complete precipitation of the ions as Al(OH), and Co(OH),. The total mass of the precipitate is 22.83 g. Find the masses of Al+ and Co+ in the solution. mass of Al3+. mass of Co+.arrow_forwardWhen barium acetate dissolves in water, what ions form? A. Ba2+ + C2H3COO- B. Ba+ + C2H3COO-2 C. B2+ + C2H3COO- D. Barium Acetate isn't solublearrow_forward9. Calculate the mass of silver phosphate solid that will precipitate when 50.0 ml. of 0.200 M silver nitrate solution is mixed with 30.0 mL of 0.250 M sodium phosphate. The other product is aqueous sodium nitrate. First write a balanced chemical equation! M Inbox Netitiontionsarrow_forward
- What can be added to a solution of Mg2+ ions to precipitate the ions out of solution? a. potassium sulfateb. ammonium carbonatec. iron (III) nitrated. all of the abovee. none of the abovearrow_forward14. Which of the following correctly describes one or more of the differences between a strong and weak electrolyte? a. A strong electrolyte partially ionizes in solution and a weak electrolyte completely ionizes in solution. b. Strong electrolytes are all classified as soluble ionic substances and weak electrolytes are all classified as soluble molecular substances. c. Strong electrolytes produce more ions per mole of substance in solution than weak electrolytes. d. Weak electrolytes inhibit the flow of electricity. e. Strong electrolytes are weak conductors of electricity.arrow_forwardThe addition of 0.3800 L of 1.150 M KCl to a solution containing Ag+ and Pb? + ions is just enough to precipitate all of the ions as AgCl and PbCl,. The total mass of the resulting precipitate is 61.40 g. Find the masses of PbCl, and AgCl in the precipitate. mass of PbCl,: mass of AgCl:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY