
Chemistry for Engineering Students
4th Edition
ISBN: 9781337398909
Author: Lawrence S. Brown, Tom Holme
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Question 7 please
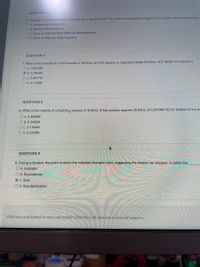
Transcribed Image Text:QUESTION 6
6. Consider a solid solute being dissolved into a liquid solvent. The combined processes of agitating the solution and pulverizing the
O A. Increase dissolving time
O B. Reduce dissolving time
OC. Have an unpredictable effect on dissolving time
O D. Have no effect on dissolving time
QUESTION 7
7. What is the molarity of a KOH solution if 38,65mL of KOH solution is required to titrate 25.84mL of 0,1982M HCI solution?
O A. 0.0015M
O B. 0.2963M
O C. 0.8817M
O D. 0.1325M
QUESTION 8
8. What is the molarity of a Ba(OH)2 solution if 18,62mL of this solution requires 35,84mL of 0.2419M HCI for titration to the ec
O A. 0.4656M
O B. 0.3492M
OC. 0.1164M
O D. 0.2328M
QUESTION 9
9. During a titration, the point at which the indicator changes color, suggesting the titration be stopped, is called the
O A. Indicator
O B. Equivalence
O C. End
O D. Standardization
Click Save and Submit to save and submit. Click Save All Answers to save all answers.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- . Which of the following will affect the total amount of solute that can dissolve in a given amount of solvent? a. The solution is stirred. b. The solute is ground to line particles before dissolving. c. The temperature changes.arrow_forwardThe equation for a reaction by which a solution of sodium carbonate may be standardized is 2HC7H5O2+Na2CO32NaC7H5O2+H2O+CO2. A student determines that 5.038g of HC7H5O2 uses 51.89mL of sodium carbonate solution in the titration. Find the molarity of the sodium carbonate.arrow_forwardWhich of the following will affect the total amount of solute that can dissolve in a given amount of solvent? a. The solution is stirred. b. The solute is ground to fine particles before dissolving. c. The temperature changes.arrow_forward
- If 55 mg of lead(II) sulfate is placed in 250 mL of pure water, does all of it dissolve? If not, how much dissolves?arrow_forwardThe solubility product constant for calcium oxalate is estimated to be 4 109. What is its solubility in grams per liter?arrow_forwardConsider the following two acids: In two separate experiments the pH was measured during the titration of 5.00 mmol of each acid with 0.200 M NaOH. Each experiment showed only one stoichiometric point when the data were plotted. In one experiment the stoichiometric point was at 25.00 mL added NaOH, and in the other experiment the stoichiometric point was at 50.00 mL NaOH. Explain these results. (See Exercise 113.)arrow_forward
- 12.49 The Safe Drinking Water Act of 1974 established the maximum permitted concentration of silver ion at 0.05 ppm. What is the concentration of Ag+ in parts per million in a saturated solution of AgCl? (NOTE: 1 ppm = 1 mg of solute/L of solution.)arrow_forwardA solution contains 0.00740 M calcium ion. A concentrated sodium fluoride solution is added dropwise to precipitate calcium fluoride (assume no volume change). a At what concentration of F does precipitate start to form? b When [F] = 9.5 104 M, what is the calcium-ion concentration? What percentage of the calcium ion has precipitated?arrow_forwardWhen methyl red is added to an aqueous solution, a pink color results. When methyl orange is added to the same solution, a yellow color is produced. What is the approximate pH range of the solution? Use Figure 18.24arrow_forward
- Consider a 1.50-g mixture of magnesium nitrate and magnesium chloride. After dissolving this mixture in water, 0.500 M silver nitrate is added dropwise until precipitate formation is complete. The mass of the white precipitate formed is 0.641 g. a. Calculate the mass percent of magnesium chloride in the mixture. b. Determine the minimum volume of silver nitrate that must have been added to ensure complete formation of the precipitate.arrow_forwardDescribe how the amount of sodium hydroxide in a mixture can be determined by titration with hydrochloric acid of known molarity.arrow_forwardCalculate the enthalpy of solution ( H for the dissolution) per mole of CaCl2 (refer to exercise 25).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning
